BLD Insights
The Expansion of Nucleoside Drugs - Chemical Modifications of the Bases
30 September 2021
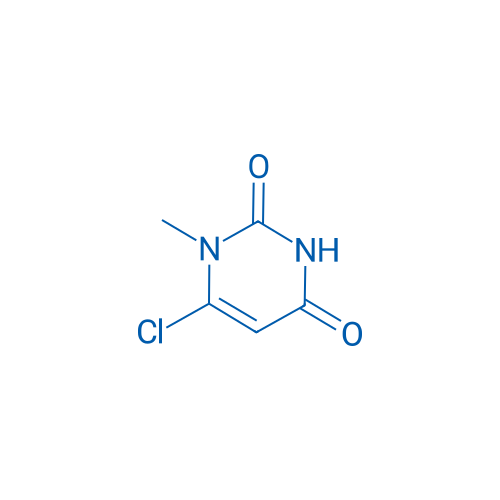
6-Chloro-1-methylpyrimidine-2,4(1H,3H)-dione
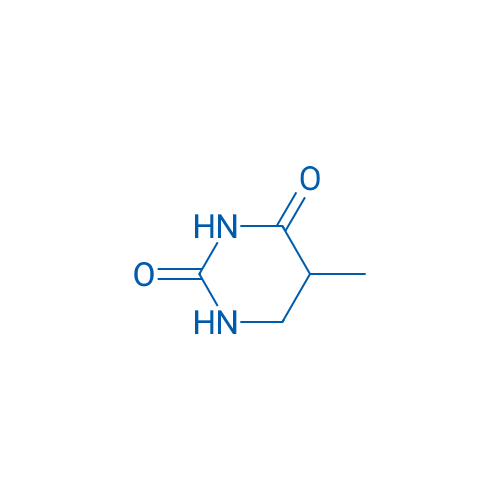
5-Methyldihydropyrimidine-2,4(1H,3H)-dione
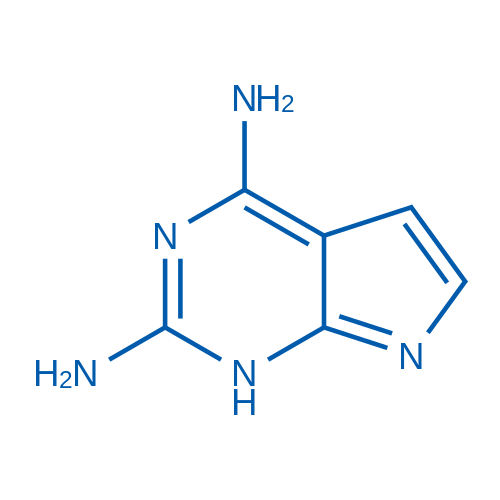
1H-Pyrrolo[2,3-d]pyrimidine-2,4-diamine
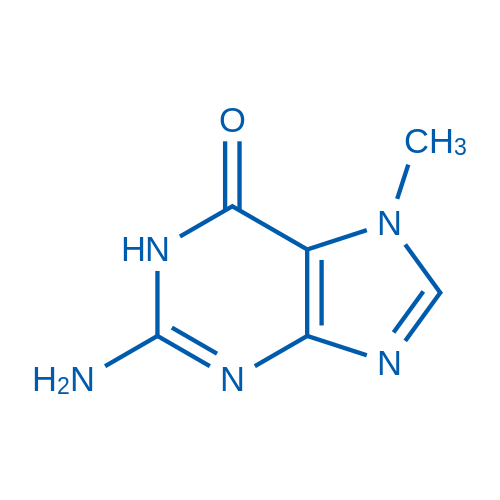
2-Amino-7-methyl-1H-purin-6(7H)-one
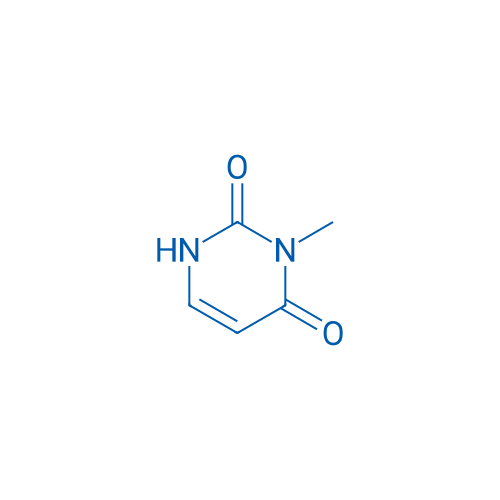
3-Methylpyrimidine-2,4(1H,3H)-dione
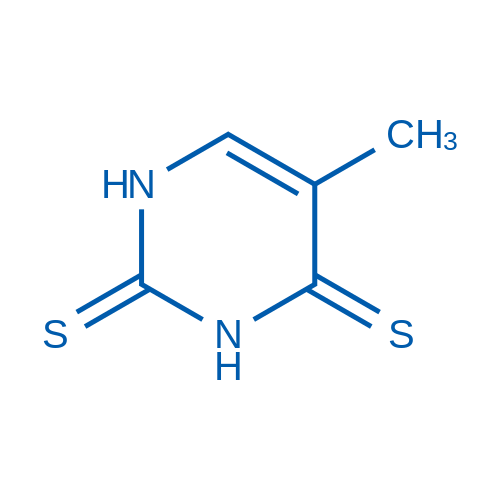
5-Methylpyrimidine-2,4(1H,3H)-dithione
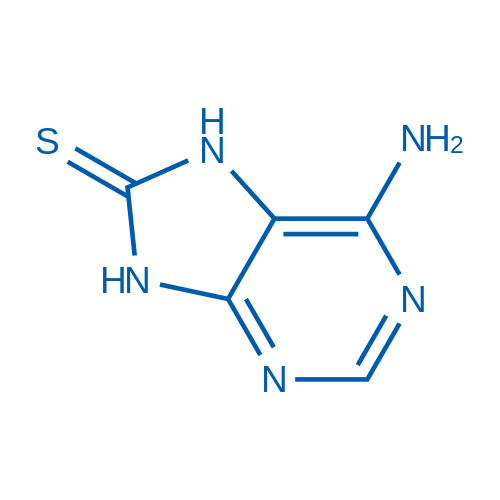
6-Aminopurine-8(9H)-thione
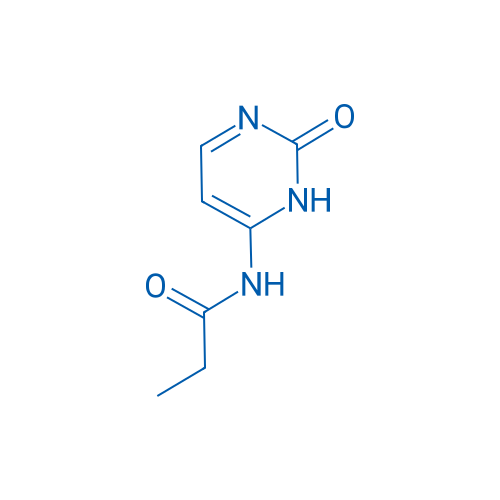
N-(2-Oxo-2,3-dihydropyrimidin-4-yl)propionamide
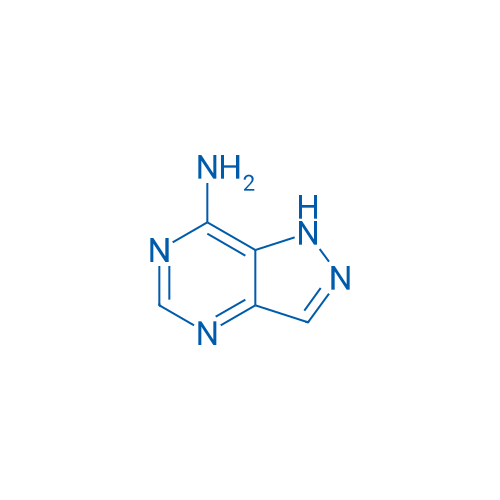
1H-Pyrazolo[4,3-d]pyrimidin-7-amine
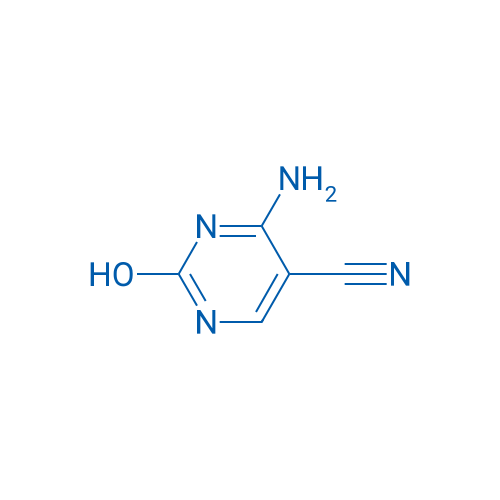
4-Amino-2-hydroxypyrimidine-5-carbonitrile

6-Chloro-1-methylpyrimidine-2,4(1H,3H)-dione

5-Methyldihydropyrimidine-2,4(1H,3H)-dione

1H-Pyrrolo[2,3-d]pyrimidine-2,4-diamine

2-Amino-7-methyl-1H-purin-6(7H)-one
Nucleoside drugs are widely used clinically because of their great antiviral and antitumor efficacy. Increasing studies are trying to modify nucleoside by chemical methods to expand its potentials for therapeutic applications, of which chemical modification of bases is one of the most common methods. There have been many examples to improve the efficacy of nucleoside drugs, alter their modes of action, or reduce toxic side effects, etc.
Clinical application of nucleoside analogs
A nucleoside is composed of a sugar moiety and nucleobase, whereas a nucleotide is composed of a sugar, nucleobase, and at least one phosphate (or phosphate-like) group(Figure 1)(Yates and Seley-Radtke, 2019). Nucleosides play a key role in biology processes, involving in the retention, replication and transcription of gene information (Lin et al., 2021). Nucleoside analogues have been widely used clinically, showing good antiviral and anti-tumor effects.
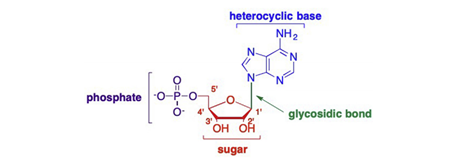
Figure 1
Chemical modification of nucleoside bases
There are mainly five common nucleobases (Figure 2), which are divided into purine bases and pyrimidine bases. Nucleobase modification is required for biology processes in eukaryotic cells. For instance, post-transcriptional modification of RNA is a universal event in molecular biology, which is methylated and deaminated by methyltransferases (MTase) and deaminases to modify nucleobases, respectively. The modified nucleosides can alter RNA structure by affecting base stacking potential, or favoring a specific nucleotide conformation, affect its function, cellular localization, and intracellular transport of this biopolymer (Flamme et al., 2019). In addition to these naturally modifications, increasing studies are trying to modify bases by chemical methods to expand its potentials for therapeutic applications. For example, the development of synthetic mRNA drug holds great promise but lies behind small molecule and protein drugs due to the challenging issues regarding its stability, immunogenicity and potency. Chemical modifications endow the synthetic mRNAs with high stability and reduced stimulation of innate immunity (Gao et al., 2021).
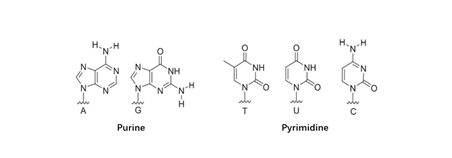
Figure 2
At present, there have been many attempts for chemical modification of nucleoside bases. 2-site and 6-site of the purine can be usually modified for purine nucleoside analogues. For example, the modification of 6-site of 6MMPr( anti-Zika virus) show more lipophilic (Figure 3); as for the modification of 2-site, Nairabine is effective against T-cell malignant tumor (T-LBL) and T-cell leukemia (T-ALL), and Cladribine, Clarabine and Fludarabine are suitable for leukemia or lymphoma(Figure 4); the number of N could also be changed, glycosidic bonds could be transformed from traditional C-N bonds to "C-C" bonds, and BCX-4430(broad-spectrum antiviral capability)and Remdesivir belongs into this type of nucleoside(Figure 5). Similarly, pyrimidine nucleoside analogs can also be modified at different positions to change the structure of the ring, improve the efficacy, alter the mode of action of the drug, or reduce toxic side effects, etc(Figure 6). Beyond that, there are other nucleoside analogs derived from triazoles, which are also widely used in clinic (Lin et al., 2021).
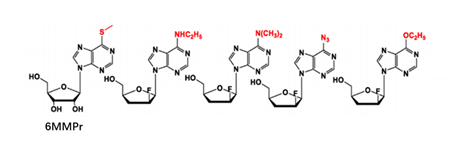
Figure 3
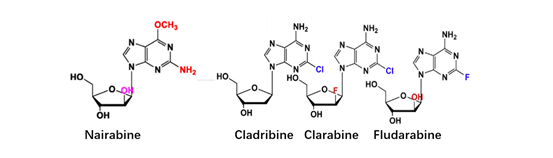
Figure 4
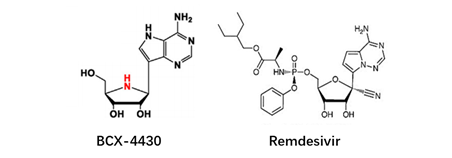
Figure 5
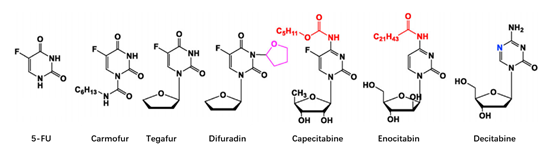
Figure 6
References
[1]Flamme, M., McKenzie, L.K., Sarac, I., and Hollenstein, M. (2019). Chemical methods for the modification of RNA. Methods 161, 64-82.
[2]Gao, M., Zhang, Q., Feng, X.H., and Liu, J. (2021). Synthetic modified messenger RNA for therapeutic applications. Acta Biomater 131, 1-15.
[3]Lin, X., Liang, C., Zou, L., Yin, Y., Wang, J., Chen, D., and Lan, W. (2021). Advance of structural modification of nucleosides scaffold. Eur J Med Chem 214, 113233.
[4]Yates, M.K., and Seley-Radtke, K.L. (2019). The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold. Antiviral Res 162, 5-21.