BLD Insights
25.10.2021
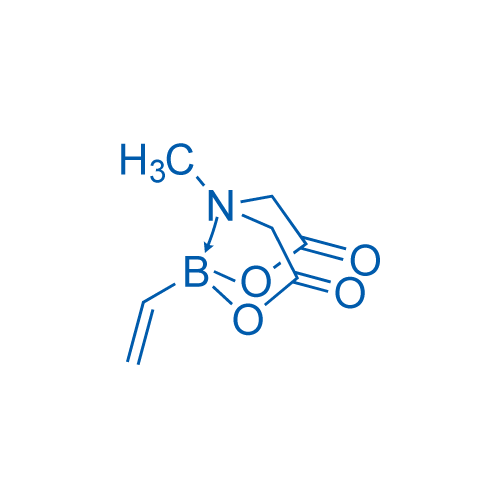
6-Methyl-2-vinyl-1,3,6,2-dioxazaborocane-4,8-dione
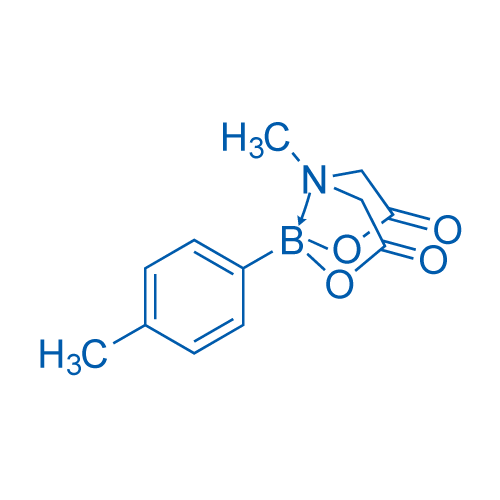
(T-4)-[N-[(Carboxy-κO)methyl]-N-methylglycinato(2-)-κN,κO](4-methylphenyl)boron
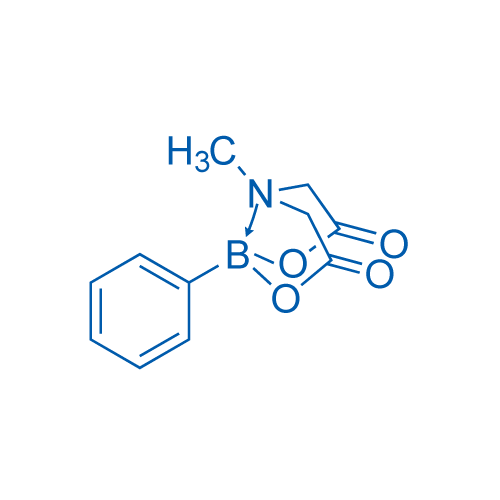
Phenyl[N-methyliminodiacetato-O,O',N]borane
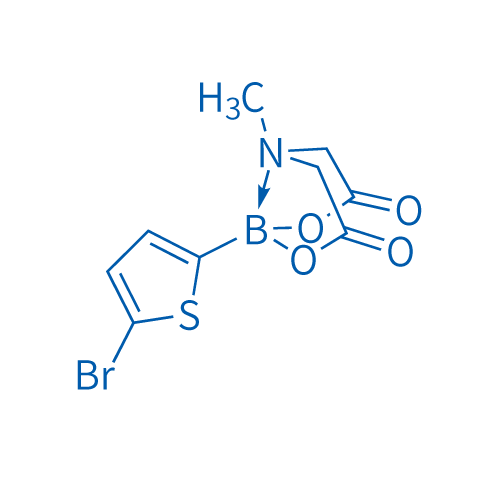
5-Bromo-2-thiophenylboronic acid mida ester
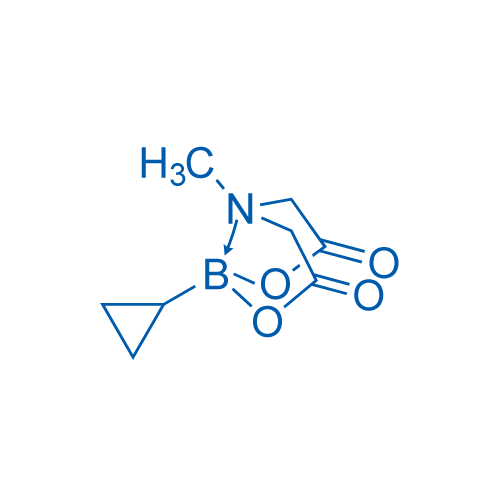
2-Cyclopropyl-6-methyl-1,3,6,2-dioxazaborocane-4,8-dione
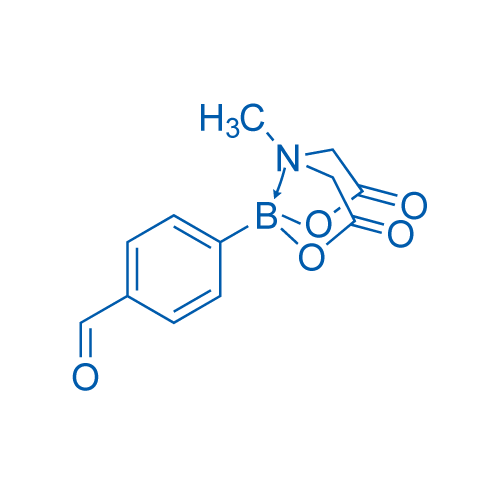
4-Formylphenylboronic acid MIDA ester
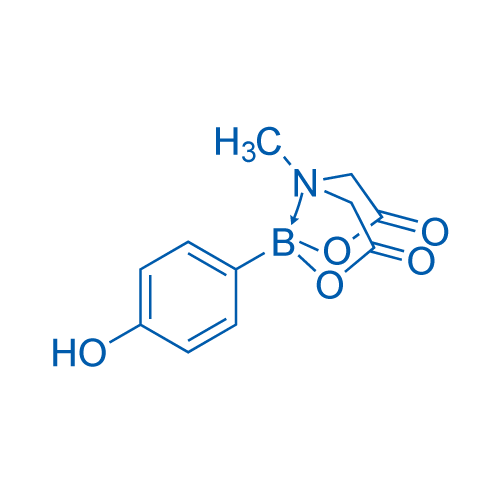
4-Hydroxyphenylboronic acid MIDA ester

6-Methyl-2-vinyl-1,3,6,2-dioxazaborocane-4,8-dione

(T-4)-[N-[(Carboxy-κO)methyl]-N-methylglycinato(2-)-κN,κO](4-methylphenyl)boron

Phenyl[N-methyliminodiacetato-O,O',N]borane

5-Bromo-2-thiophenylboronic acid mida ester
N-methyliminodiacetic acid (MIDA) boronates are potential alternative nucleophiles of traditional boronic acid agents for Suzuki cross-coupling reaction (Figure 1). MIDA boronates have excellent tolerance to air, moisture and silica gel are usually indefinitely stable on the benchtop as free-flowing crystalline solid, which offers chemists desirable convenience in the separation and purification process of such structures through silica gel column chromatography or recrystallization. Besides, MIDA boronates are liable to perform deprotection reactions under a weak base environment to give boronic acid. Thus, MIDA boronates have been widely applied as building blocks in the synthesis of small molecules with various structures, including natural products, pharmaceuticals precursors and polymers1.
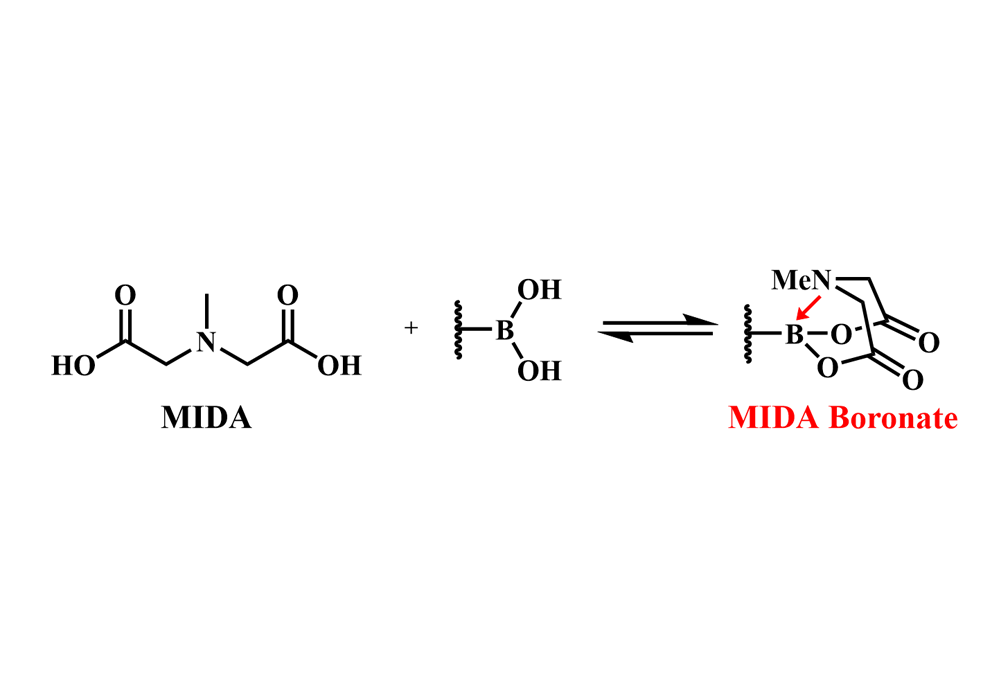
Figure 1
Small molecules synthesis with MIDA boronates involved iterative cross-coupling
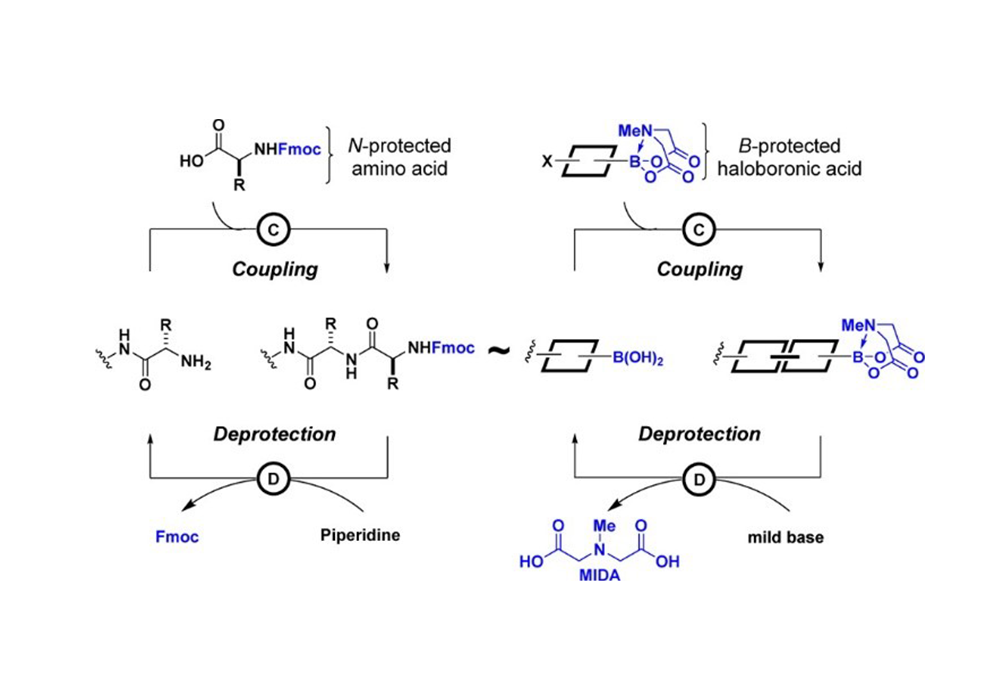
Figure 2
Based on the iterative cross-coupling (ICC) reaction with bi-functional halo-boronic acid as building blocks, one is capable of expediently preparing a great diversity of small molecules including natural products, drug molecules and synthetic material precursors (Figure 2)2. Experimental evidence has shown that such bifunctional reagents exhibit remarkable practical application value in the synthesis of poly-ene types natural products such as Ratanhine and Peridinin; drug candidate C35deOAmB (a derivative of Amphotericin B) as well as Secodaphnane Core (a key intermediate for the synthesis of daphniphylum alkaloids) (Figure 3).
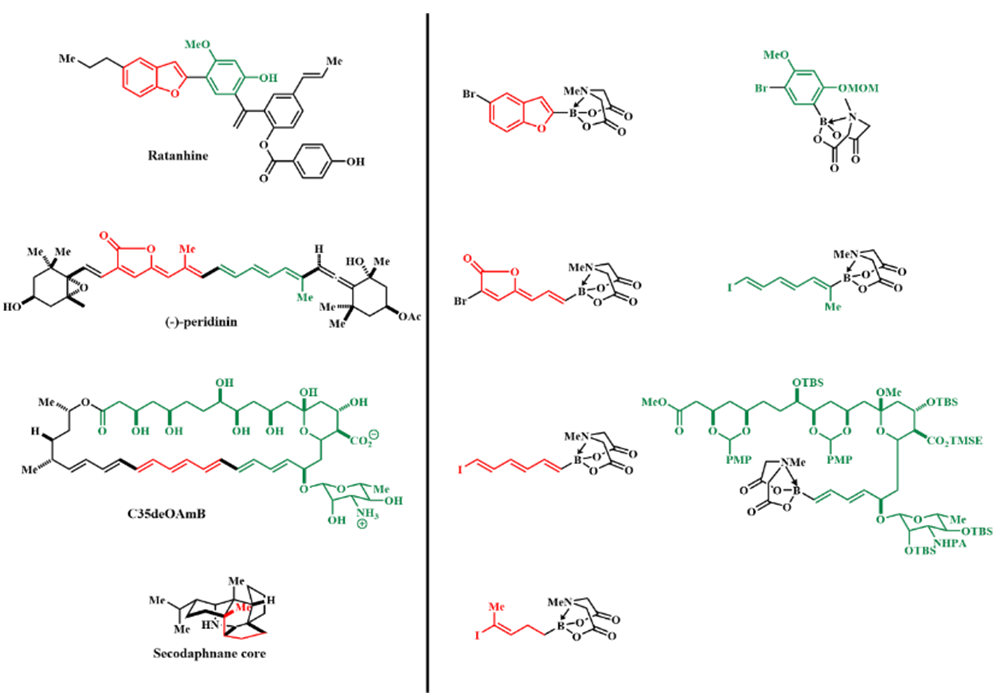
Figure 3
Polymers synthesis from MIDA boronate-containing monomers
N-methyliminodiacetic acid boronates could keep intact under eversible addition-fragmentation chain transfer (RAFT) polymerization conditions, which guaranteed the applicability of MIDA boronate-containing monomers in the preparation of modifiable boracic polymers. In virtue of their excellent feasibility to perform deprotection and Suzuki cross-coupling reactions, such MIDA boronate-containing polymers are suitable to act as versatile platforms to prepare functionalized polymers3 (Figure 4).
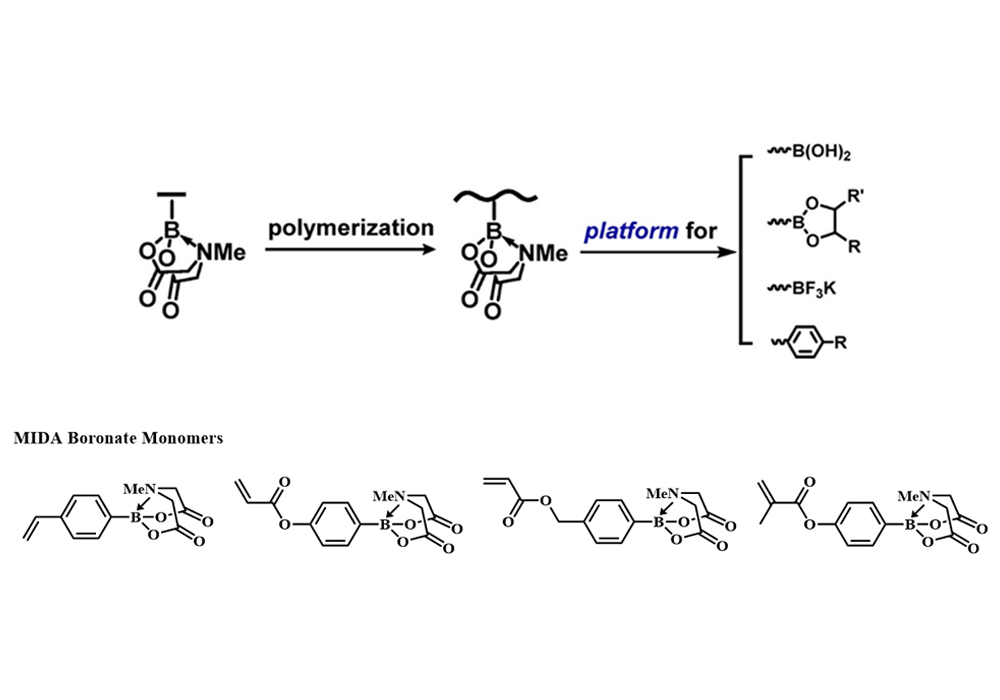
Figure 4
In addition, there is a significant difference in the solubility of MIDA boronates compared with corresponding boronic acid, which enabled the rapid separation of MIDA boronate products. Therefore, when bifunctional haloboronic acid is employed as building blocks, regio- and sequence-defined polymers could be readily prepared through ICC4 (Figure 5).
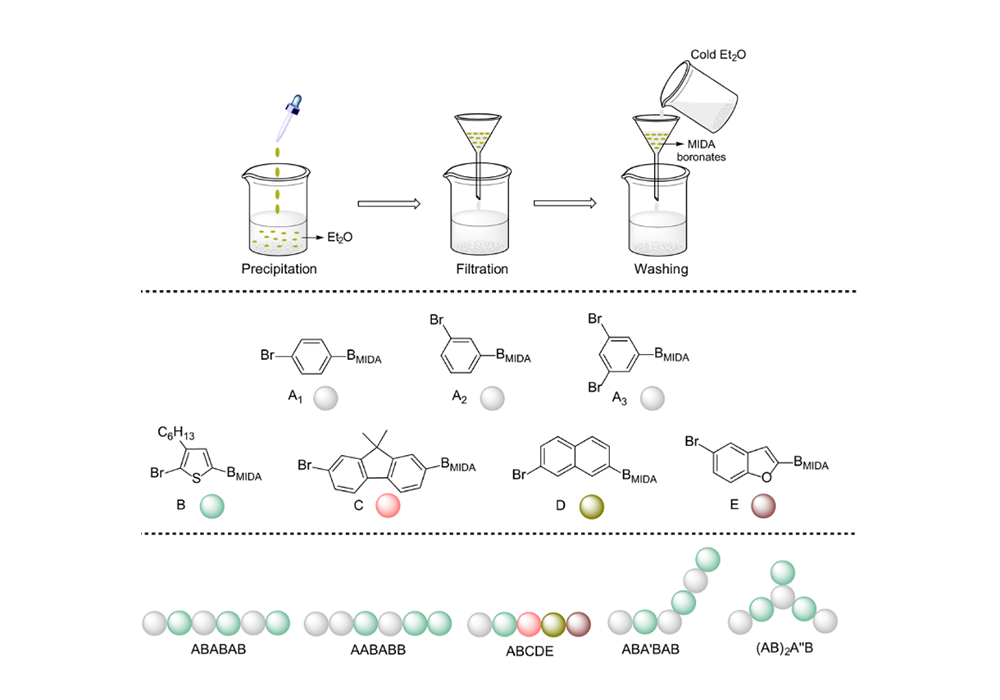
Figure 5
Functionalized derivative of MIDA boronate
Adjusting the substituent group on N atom of MIDA could endow them with multiple functions including acting as a chiral auxiliary in diastereoselective reactions5(Figure 6) or directing group for C-H functionalization6 (Figure 7).
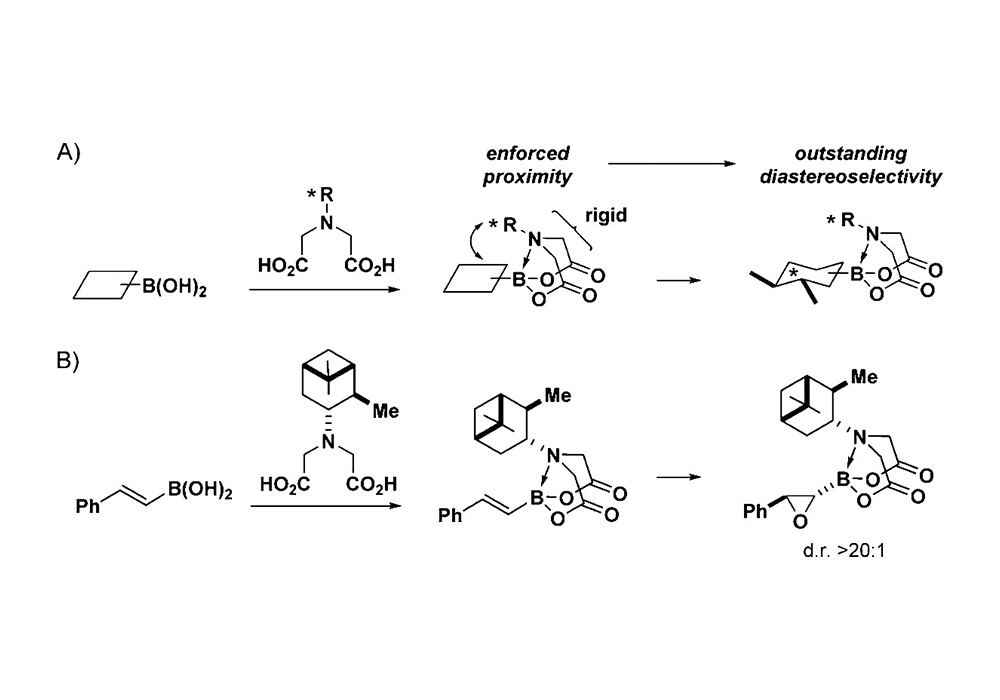
Figure 6
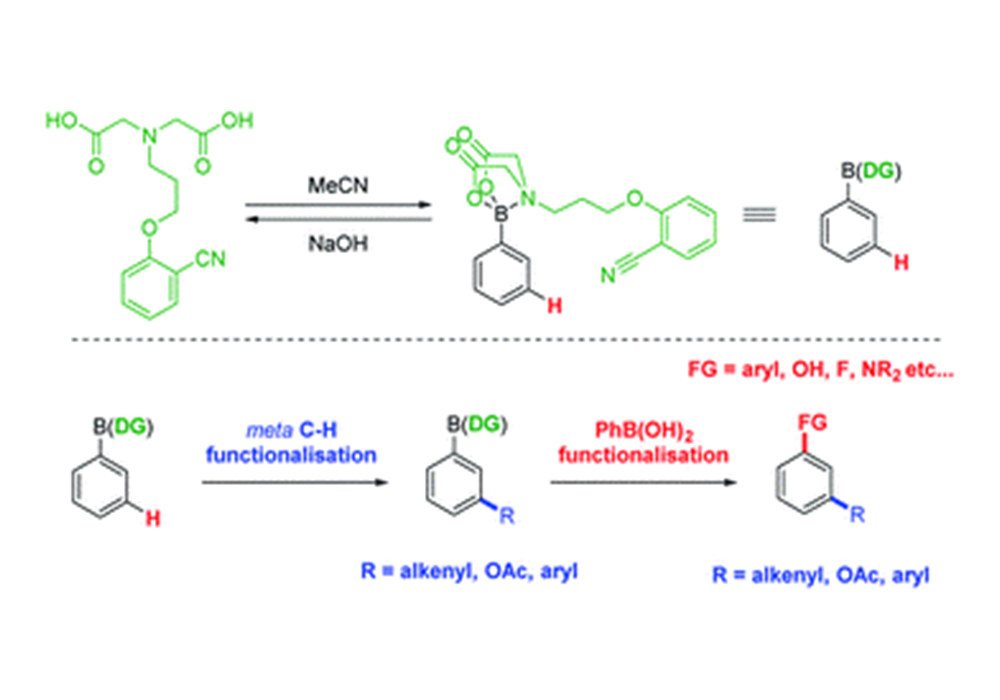
Figure 7
References