BLD Insights
New Breakthrough: Generate pluripotent stem cells via chemical reprogramming of human somatic cells
13 May 2022
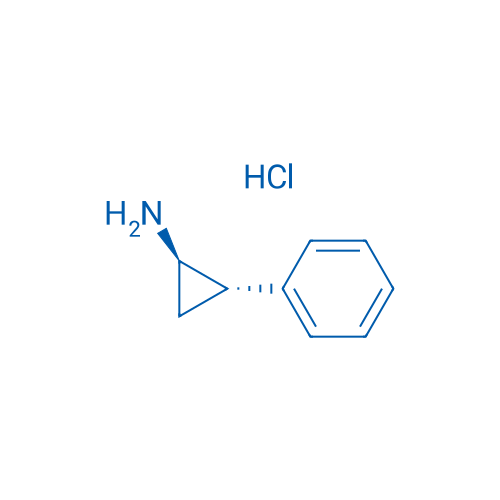
trans-2-Phenylcyclopropanamine hydrochloride
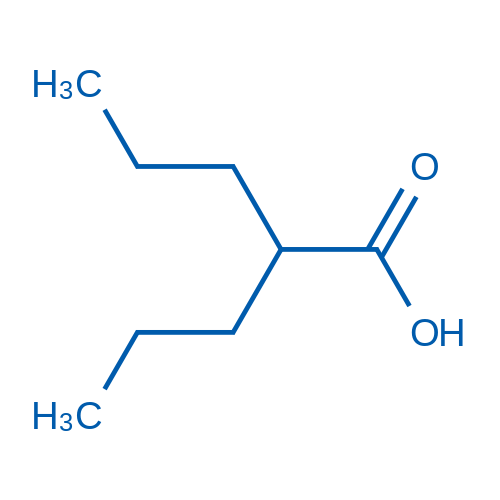
2-Propylpentanoic acid
6,7-Dimethoxy-2-(pyrrolidin-1-yl)-N-(5-(pyrrolidin-1-yl)pentyl)quinazolin-4-amine
4-(4-(Benzo[d][1,3]dioxol-5-yl)-5-(pyridin-2-yl)-1H-imidazol-2-yl)benzamide

trans-2-Phenylcyclopropanamine hydrochloride

2-Propylpentanoic acid
To obtain pluripotent stem cells through easy and safe way has always been valuable research. In 2013, Deng Hongkui's group published the article Pluripotent Stem Cells Induced from Mouse Somatic Cells by Small-Molecule Compounds, which validated "a whole new route" to get pluripotent stem cells by inducing a pluripotent state in mouse somatic cells with a combination of seven small-molecule compounds. Compared with the method of Shinya Yamanaka's group, via which they generated induced pluripotent stem cells through expression of a cocktail of factors (Oct3/4, Sox2, c-Myc, and Klf4), small-molecule compounds are non-integrative to the genome, highly controllable, and easy to optimize, standardize and manufacture. This makes chemical induced pluripotent stem cells (CiPSCs) show more attractive in application. However, for the fact that human somatic cells have evolved to have a more stable epigenetic landscape to protect its cell identities from chemical-based perturbation, researchers failed to induce human pluripotent stem cells from somatic cells in the past few years.
April 13, again, Deng Hongkui's group published the article
The researchers thought they may get induction of the intermediate plastic state at the early stage, during which chemical-induced dedifferentiation occurred, and this process was similar to the dedifferentiation process that occurs in axolotl limb regeneration.
The whole chemical reprogramming trajectory analysis delineated the induction of the intermediate plastic state at the early stage, during which chemical-induced dedifferentiation occurred, and this process was similar to the dedifferentiation process that occurs in axolotl limb regeneration. To prove this, they have tested millions of groups of small molecule compounds in the past years, to get hCiPSCs through human somatic cells, and described the reprogramming trajectory of this intermediate plastic state as below (Figure 1):
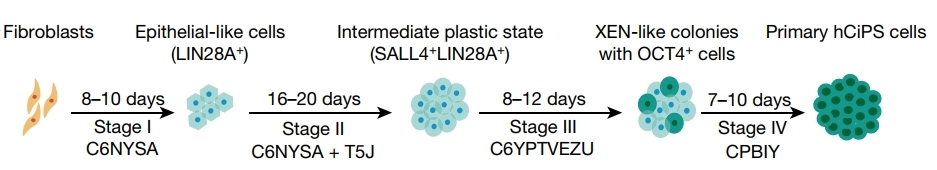
Figure 1
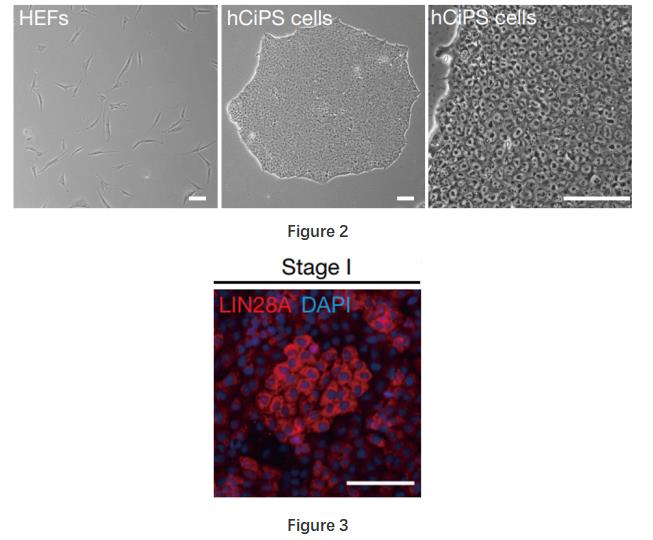
Stage 1 They identified a small molecule combination (CHIR99021 (10 μM), 616452 (10 μM), TTNPB (2 μM), SAG (0.5 μM), ABT-869 (1 μM), Y-27632 (2 μM))that converted human fibroblasts into epithelial-like cells (Figure 2). Consistently, this treatment upregulated epithelial cell-related genes (KRT8, KRT18 and KRT19) and downregulated a panel of fibroblast marker genes, suggesting a loss of fibroblast identity. LIN28A, an important gene that regulates dedifferentiation and regeneration in different species was expressed during this stage (Figure 3).
Stage 2 They identified a small molecule combination (CHIR99021 (10-12 μM), 616452 (10 μM), TTNPB (2 μM), SAG (0.5 μM),ABT-869 (1 μM), Y27632 (10 μM)) could maintain the culture. The addition of JNKIN8 (1 μM, c-Jun N-terminal kinase inhibitor) combined with 5-azacytidine (10 μM) and tranylcypromine (10 μM) was able to induce hypomethylation and a proliferation cell state. Inhibition of pro-inflammatory pathway through JNK inhibitor, JNKIN8, is essential for cell proliferation, as well as for the open chromatin status of the genes activated by the end of stage 2. Note: the researchers suggested the addition of UNC0224 (1 μM), ruxolitinib (1 μM) and SGC-CBP30 (2 μM) in cell culture during stage 2.
These cells also acquired a hypomethylated epigenetic state at stage II, and the promoter regions of genes related to embryonic development, cell cycle and stem cell proliferation were demethylated, suggesting characteristics of cell dedifferentiation and a plastic cell state. Meanwhile, the researchers identified a series of key genes, including LIN28A, SALL4 (Figure 4), genes involved in maintaining cell proliferation and their downstream (Figure 5), expressed, and MSX1/MSX2/HOXB9, three developmental regulators, transiently activated in the plastic intermediate state (Figure 6).
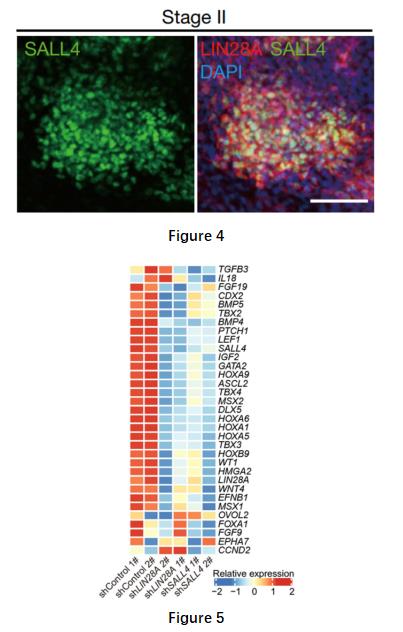
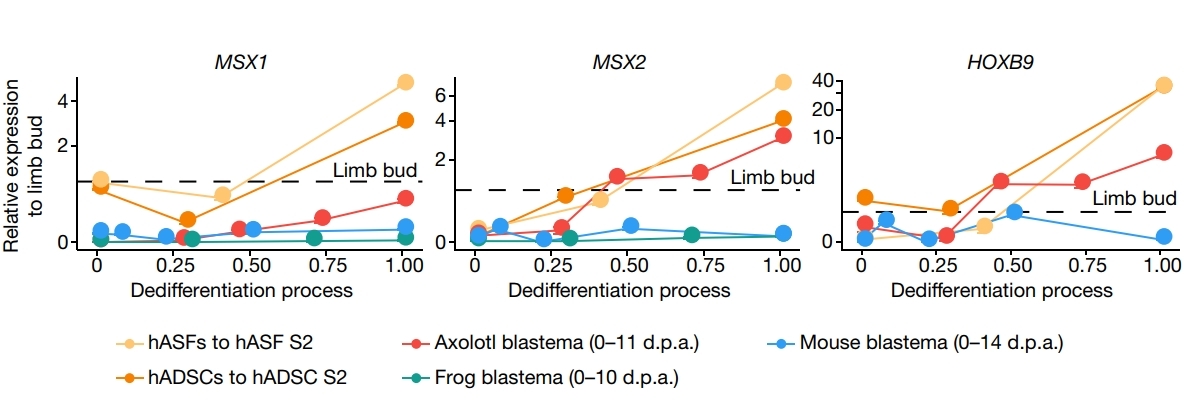
Figure 6
Stage 3: The use of a small-molecule combination, including epigenetic regulators (tranylcypromine (10 μM), VPA (500 μM), DZNep (0.2 μM), EPZ004777 (5 μM), UNC0379 (1 μM)) and cell signalling inhibitors (CHIR99021, 616452, Y27632 and PD0325901) activated expression of OCT4 (Figure 7), a key transcriptional regulator of stem cell. OCT4 can not only maintain the undifferentiated state of stem cells, but also control the specific differentiation of them, so it has a role in determining the fate of stem cells. The expression of gene SOX17, HNF1B and EPCAM could also improve the generation of hCiPS cells.
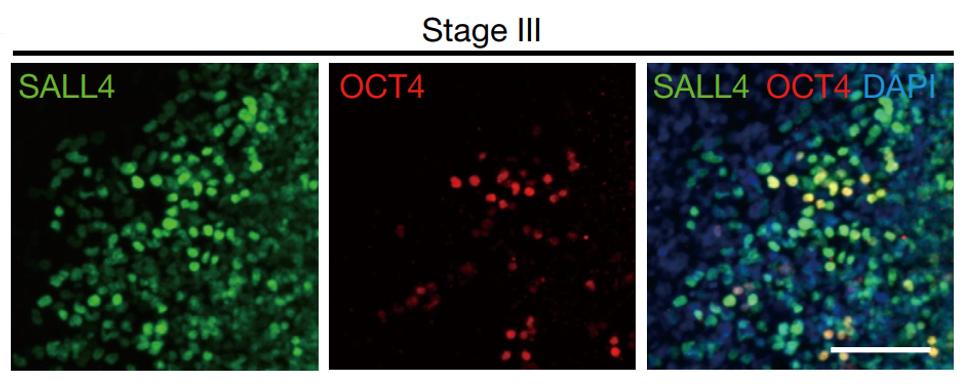
Figure 7
Stage 4: The usage of compound group (CHIR99021 (1 μM), Y-27632 (10 μM), PD0325901 (1 μM), IWP-2 (2 μM) and SB590885 (0.5 μM), VPA (500 μM, only for the first 4 days)) facilitated the maintenance of human pluripotent cells and activated the pluripotency gene network. Co-expressing OCT4, SOX2 and NANOG was induced by the compounds in compact colonies (Figure 8). After being transferred into human embryonic stem cell culture medium, these colonies displayed typical hES cell morphology.
Through analysis of a series of markers, including expression levels of TRA-1-60, TRA-1-81 and SSEA-4 (figure 9, a panel of pluripotency genes), and a panel of pluripotency genes, demethylation of the loci of OCT4 and NANOG promoters with an open chromatin accessibility, the researchers identified the cells at stage 4 resembled human embryo stem cells in transcriptomic and epigenetic profiles. The compact colonies were able to form cells of the three germ layers in vivo by forming teratomas in immunodeficient mice and in vitro by forming embryoid bodies (figure 10). Directed differentiation of these cells was able to generate haematopoietic progenitor cells, hepatocytes and neural stem cells. These suggested that these cells at stage 4 are similar to human embryo stem cells. Hereafter, the researchers called these cells as human chemically induced PS (hCiPS) cells.
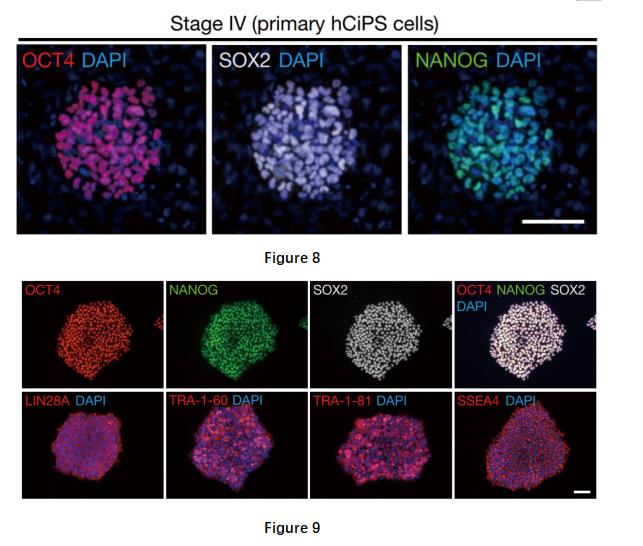
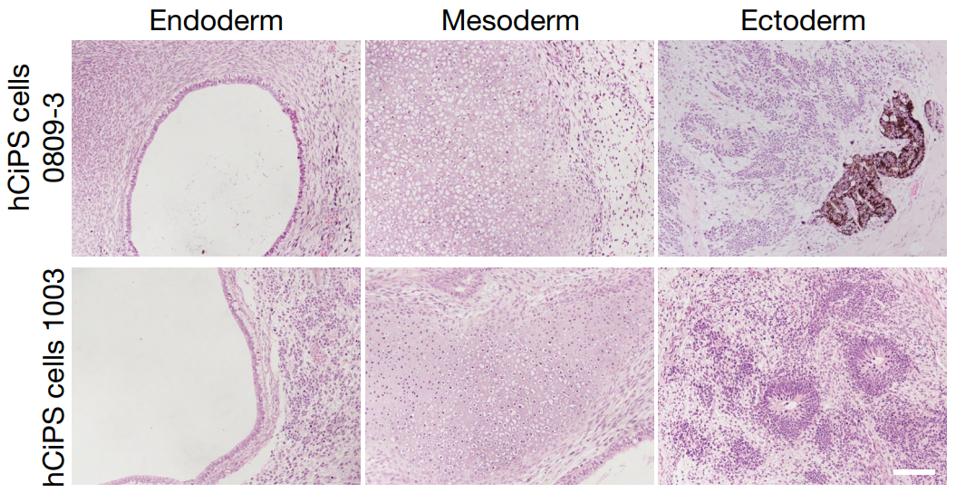
Figure 10
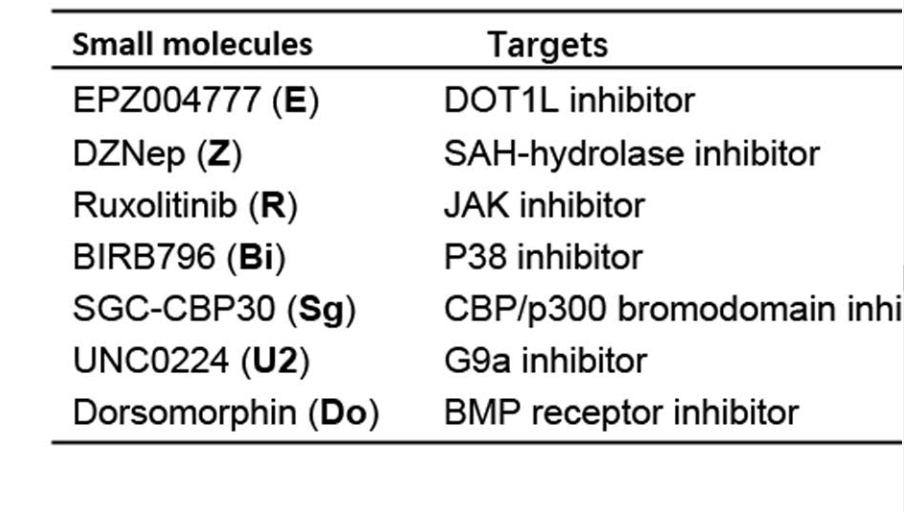
The researchers also generated hCiPS cells from adult somatic cells, including hADSCs and hASF. Small molecules used to promote the reprogramming efficiency were identified and shown as below:
References
[1]Guan J, Wang G, Wang J, Zhang Z, Fu Y, Cheng L, Meng G, Lyu Y, Zhu J, Li Y, Wang Y, Liuyang S, Liu B, Yang Z, He H, Zhong X, Chen Q, Zhang X, Sun S, Lai W, Shi Y, Liu L, Wang L, Li C, Lu S, Deng H. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature. 2022 Apr 13. doi: 10.1038/s41586-022-04593-5. Epub ahead of print. PMID: 35418683.