BLD Insights
Organic Photovoltaic Donors Based on Benzodithiophene (BDT) Blocks
01 January 2023
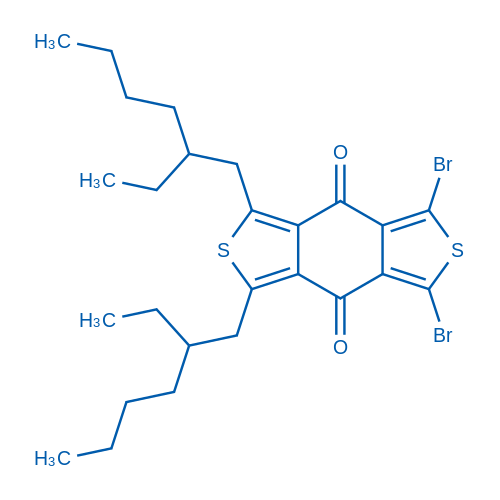
1,3-Dibromo-5,7-bis(2-ethylhexyl)-4H,8H-benzo[1,2-c:4,5-c']dithiophene-4,8-dione
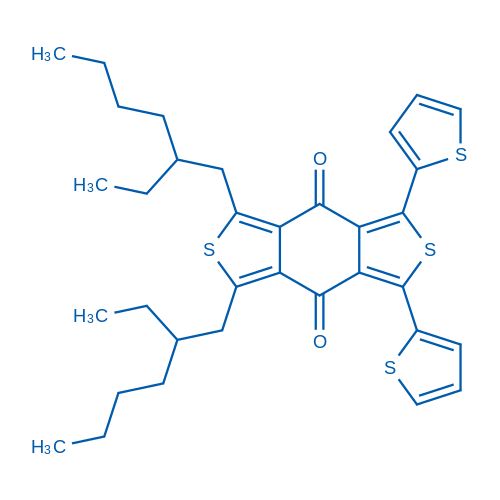
1,3-Bis(2-ethylhexyl)-5,7-di(thiophen-2-yl)benzo[1,2-c:4,5-c']dithiophene-4,8-dione
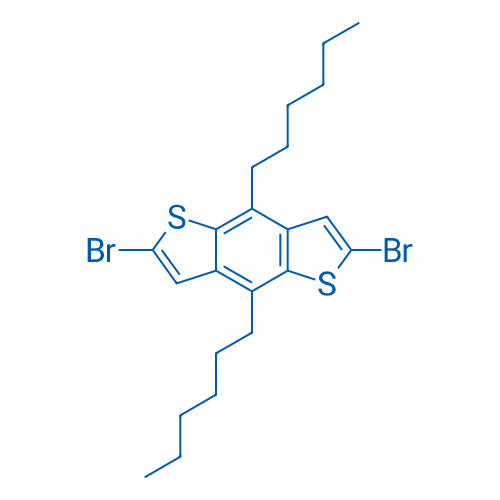
2,6-Dibromo-4,8-dihexylbenzo[1,2-b:4,5-b']dithiophene
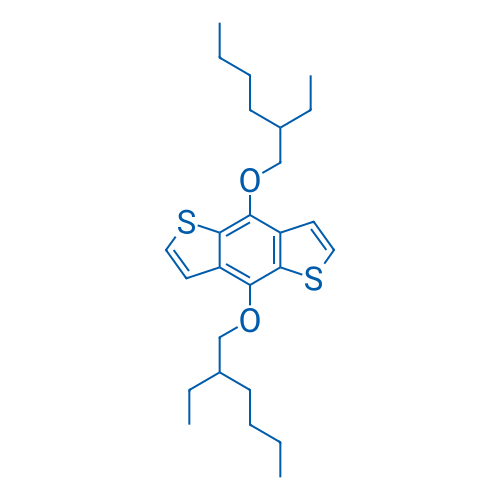
4,8-Bis((2-ethylhexyl)oxy)benzo[1,2-b:4,5-b']dithiophene
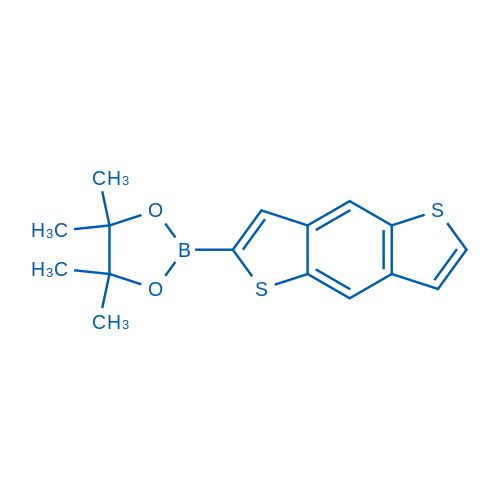
2-(Benzo[1,2-b:4,5-b']dithiophen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
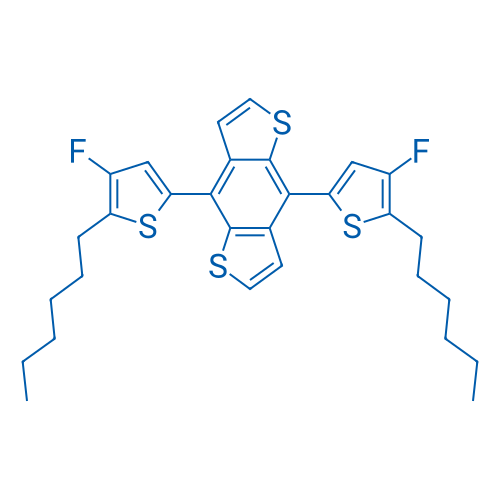
4,8-Bis(4-fluoro-5-hexylthiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene
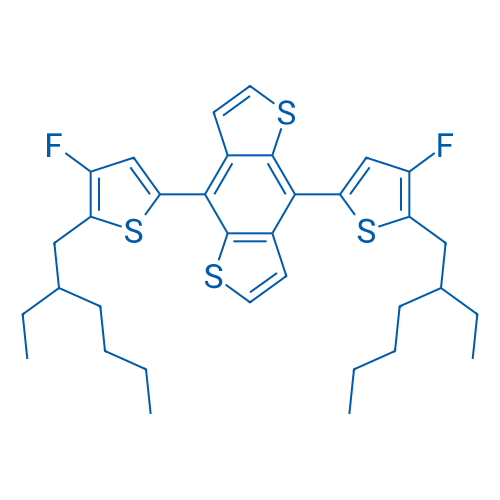
4,8-Bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)benzo[1,2-b:4,5-b']dithiophene

1,3-Dibromo-5,7-bis(2-ethylhexyl)-4H,8H-benzo[1,2-c:4,5-c']dithiophene-4,8-dione

1,3-Bis(2-ethylhexyl)-5,7-di(thiophen-2-yl)benzo[1,2-c:4,5-c']dithiophene-4,8-dione

2,6-Dibromo-4,8-dihexylbenzo[1,2-b:4,5-b']dithiophene
Benzodithiophene (BDT) unit is one of the most concerned units in constructing various D-A conjugated polymers (CPs) and small molecules, which is ascribed to its symmetric and planar conjugated structures resulting in the enhancement of π-π stacking of the molecule backbone, promoting the charge carrier mobility. Moreover, the 4 and 8 positions of BDT can be modified by different types of substituents, further optimizing the absorption, crystallinity and energy levels of the materials.
In 2010, Leclerc et al. designed and synthesized a new low-band-gap BDT-based copolymer, PBDTTPD, in which TPD was applied as the receptor of D-A copolymer to polymer photovoltaic materials. The level structure and solubility of D-A conjugated polymer were optimized by changing the alkyl side chain and adding π-bonded thiophene. The electrochemical band gap of PBDTTPD is 1.81 eV, and the PCE of PC71BM heterojunction photovoltaic device reached 5.5%. It is important to note that further improvements are possible through different device configurations such as annealing, solvents, additives, electrodes, acceptors, etc.[1] This is one of the very early exploration of utilizing BDT blocks in organic photovoltaic (OPV) field.
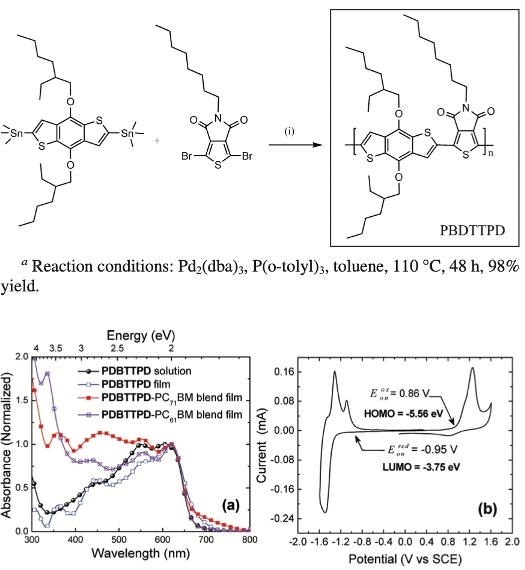
Fig. 1
The similar building block benzo[1,2-b:4,5-b']difuran (BDF) has got increasing attention due to its planar molecular structure, high mobilities, good solubility, rich sources, and no harm to the environment after degradation. Based on these advantages, benzodifuran unit has been widely used in organic photovoltaic materials. The highest power conversion efficiency (PCE) of over 10% has already been achieved based on BDF unit, revealing its great potentials in organic photovoltaic materials.
PBDFTPD polymers prepared by microwave-assisted synthesis have already achieved a PCE value of 7.4% over ten years ago in BHJ solar cells. Compared to the model polymer PBDTTPD(2EH/C8) for which blend morphologies with PCBM can be improved by addition of small-molecule additive in the blend solution (e.g. DIO, CN), the best BHJ solar cells with PBDFTPD(2EH/C8) are made without any additive, indicating that BDF is a promising donor.[2] While the relatively planar polymer PBDTTPD is more photostable than its more twisted, less-ordered benzodifuran derivative PBDFTPD.[3]
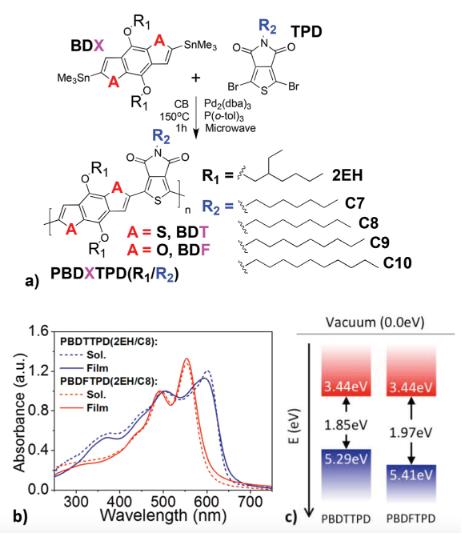
Fig. 2
Benzo[1,2-c:4,5-c′]dithiophene-4,8-dione (BDD) is one of the most popular electron-accepting building blocks for high-performance copolymers owing to its planar and symmetrical fused-ring structure and self-assembly properties.
PBDB-T congeners are mainly composed of two building blocks: BDT and BDD units. Since it was first reported, PBDB-T has shown excellent processing and photovoltaic performance. A variety of PBDB-T derivatives with excellent performance have been applied to high efficiency photovoltaic cells. Besides the spontaneous rope like morphology formation and efficient charge generation, the high PCE achieved by PBDB-T congeners shows strong competitiveness even with traditional photovoltaic techniques which significantly promotes the development of OSCs. [4]
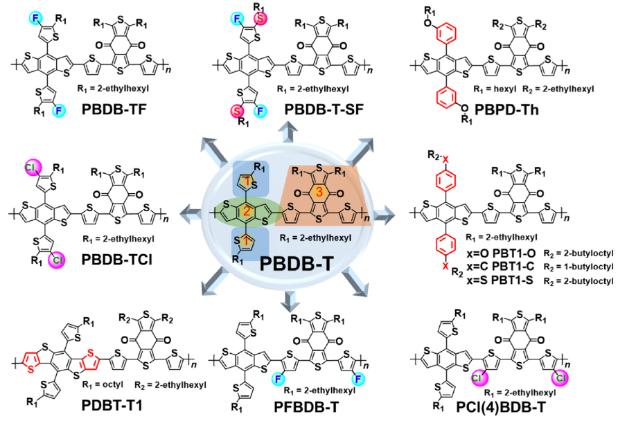
Fig. 3
Thienyl group substituted benzo[1,2-b:4,5-b′]dithiophene (BDTT), derived from BDT, has also been widely used as donor unit. In 2017, Li et al. reported a wide bandgap A−D−A structured p-type organic semiconductor (p-OS) small molecule, based on the BDTT donor unit as the core. The all-small-molecule organic solar cell (SM-OSC) with SM1 as donor and a narrow bandgap n-OS IDIC as acceptor demonstrated a high power conversion efficiency (PCE) of 10.11% and a high fill factor (FF) of 73.55, which is the highest values for the nonfullerene SM-OSCs reported in the literature at the time.[5]
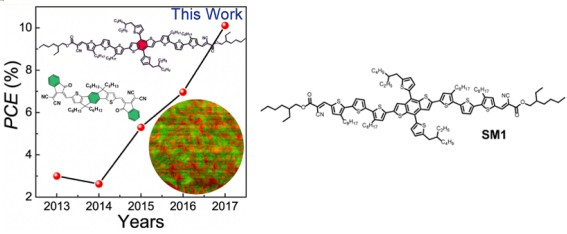
Fig. 4
References
[1]Zou, Y.; Najari, A.; Berrouard, P.; Beaupré, S.; Réda Aïch, B.; Tao, Y.; Leclerc, M., A Thieno[3,4-c]pyrrole-4,6-dione-Based Copolymer for Efficient Solar Cells. Journal of the American Chemical Society 2010, 132 (15), 5330-5331.
[2]Warnan, J.; Cabanetos, C.; Labban, A. E.; Hansen, M. R.; Tassone, C.; Toney, M. F.; Beaujuge, P. M., Ordering Effects in Benzo[1,2-b:4,5-b′]difuran-thieno[3,4-c]pyrrole-4,6-dione Polymers with >7% Solar Cell Efficiency. Advanced Materials 2014, 26 (25), 4357-4362.
[3]Mateker, W. R.; Heumueller, T.; Cheacharoen, R.; Sachs-Quintana, I. T.; McGehee, M. D.; Warnan, J.; Beaujuge, P. M.; Liu, X.; Bazan, G. C., Molecular Packing and Arrangement Govern the Photo-Oxidative Stability of Organic Photovoltaic Materials. Chemistry of Materials 2015, 27 (18), 6345-6353.
[4]Zheng, Z.; Yao, H.; Ye, L.; Xu, Y.; Zhang, S.; Hou, J., PBDB-T and its derivatives: A family of polymer donors enables over 17% efficiency in organic photovoltaics. Materials Today 2020, 35, 115-130.
[5]Qiu, B.; Xue, L.; Yang, Y.; Bin, H.; Zhang, Y.; Zhang, C.; Xiao, M.; Park, K.; Morrison, W.; Zhang, Z.-G.; Li, Y., All-Small-Molecule Nonfullerene Organic Solar Cells with High Fill Factor and High Efficiency over 10%. Chemistry of Materials 2017, 29 (17), 7543-7553.