How BLD Helps
Lenacapavir: Twice-Yearly Treatment for HIV
12 October 2022
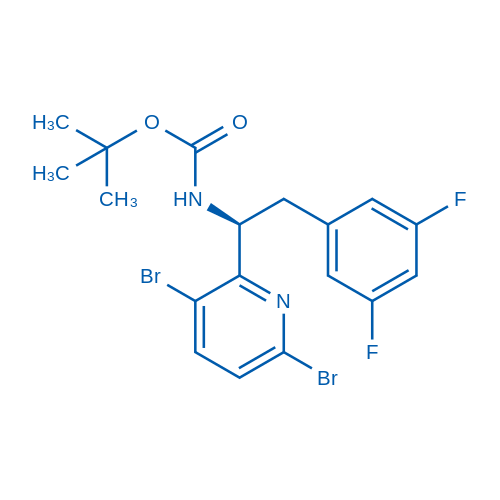
tert-Butyl (S)-(1-(3,6-dibromopyridin-2-yl)-2-(3,5-difluorophenyl)ethyl)carbamate
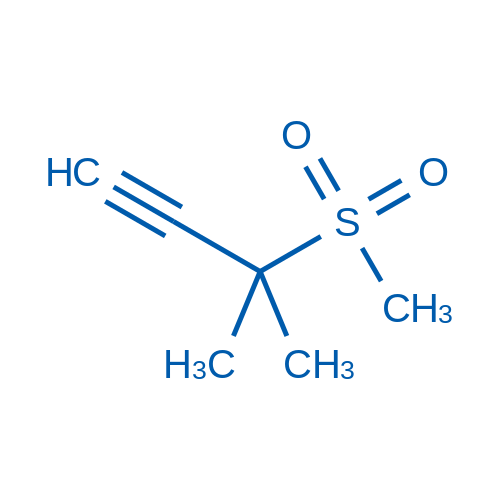
3-Methyl-3-(methylsulfonyl)but-1-yne

tert-Butyl (S)-(1-(3,6-dibromopyridin-2-yl)-2-(3,5-difluorophenyl)ethyl)carbamate

3-Methyl-3-(methylsulfonyl)but-1-yne
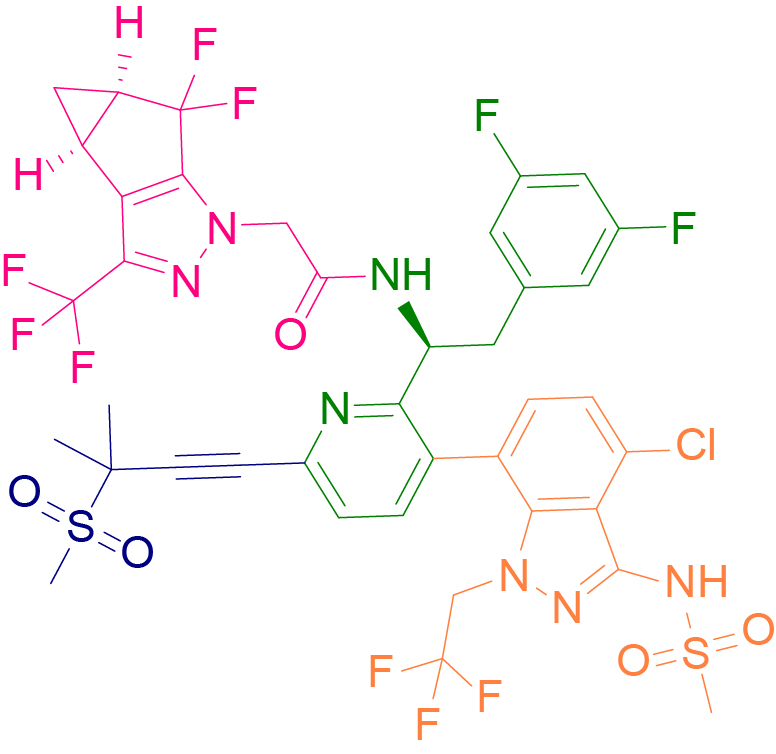
Within the HIV-1 virus, the RNA genome encoding the capsid protein is highly stable and can provide a fatal attack on the virus by inhibiting the capsid protein without rapidly developing drug resistance. Gilead's Lenacapavir is the first HIV-1 capsid protein inhibitor to treat HIV-1 infection by blocking the disassembly of the HIV-1 capsid protein. It is unique in that it can be administered subcutaneously every six months or orally once a week to provide effective treatment for HIV infection.
On August 19, 2022, Gilead announced that the European Medicines Agency (EMA) has approved the first marketing authorization application (MAA) for lenacapavir, marketed as Sunlenca®, for the treatment of adult patients with multi-drug resistant HIV-1. In Time, Gilead intends to extend the use of lenacapavir into additional and much larger indications, including the general HIV-positive population as well as for pre-exposure prophylaxis (PrEP) among people who have sex with HIV-positive partners. Analysts at RBC Capital markets recently predicted that lenacapavir could become a $4 billion blockbuster if it gets a green light across all its planned indications. On August 19, 2022, Gilead announced that the European Medicines Agency (EMA) has approved the first marketing authorization application (MAA) for lenacapavir, marketed as Sunlenca®, for the treatment of adult patients with multi-drug resistant HIV-1. In Time, Gilead intends to extend the use of lenacapavir into additional and much larger indications, including the general HIV-positive population as well as for pre-exposure prophylaxis (PrEP) among people who have sex with HIV-positive partners. Analysts at RBC Capital markets recently predicted that lenacapavir could become a $4 billion blockbuster if it gets a green light across all its planned indications.