BLD Insights
Diborons - Remains Firmer As Time Goes By!
07 October 2022
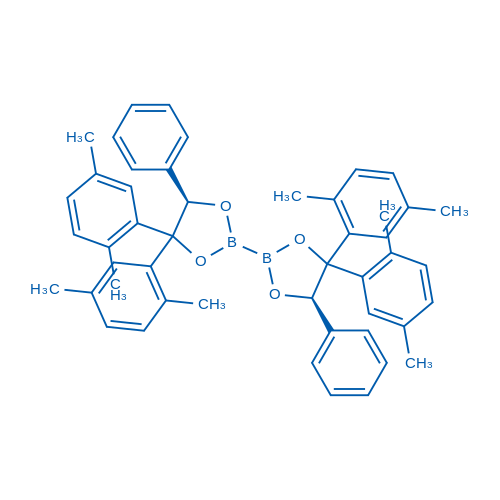
(5R,5'R)-4,4,4',4'-Tetrakis(2,5-dimethylphenyl)-5,5'-diphenyl-2,2'-bi(1,3,2-dioxaborolane)
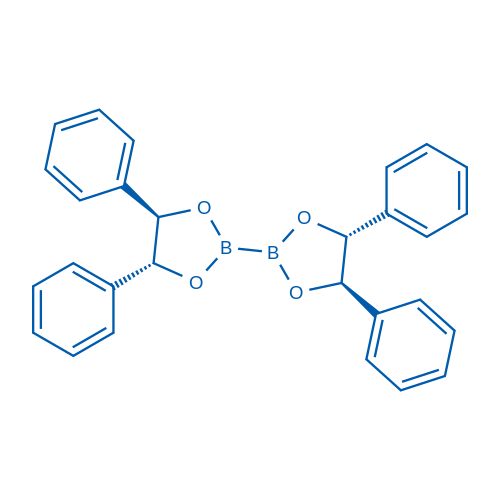
(4R,4'R,5R,5'R)-4,4',5,5'-Tetraphenyl-2,2'-bi(1,3,2-dioxaborolane)
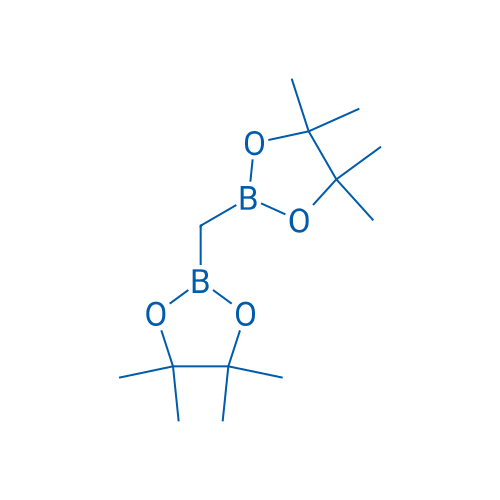
Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methane
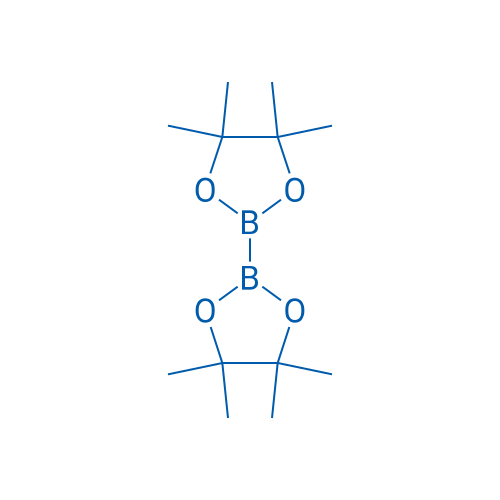
4,4,4',4',5,5,5',5'-Octamethyl-2,2'-bi(1,3,2-dioxaborolane)
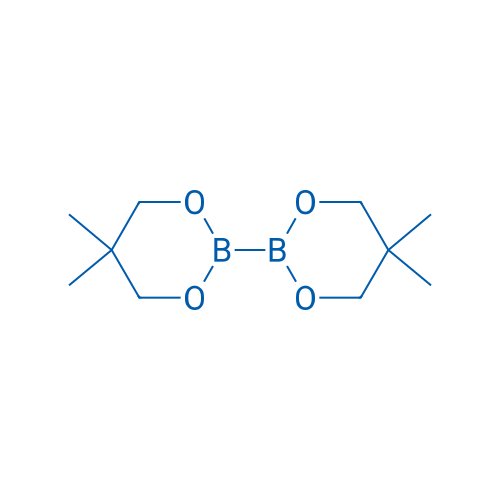
5,5,5',5'-Tetramethyl-2,2'-bi(1,3,2-dioxaborinane)
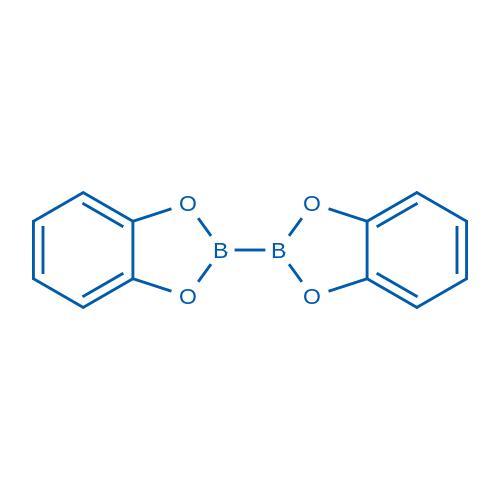
2,2'-Bibenzo[d][1,3,2]dioxaborole
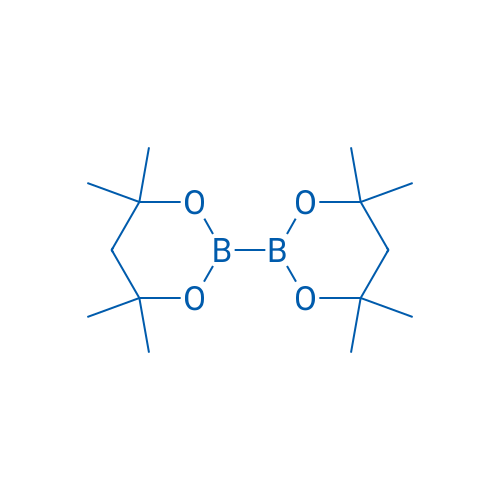
4,4,4',4',6,6,6',6'-Octamethyl-2,2'-bi(1,3,2-dioxaborinane)
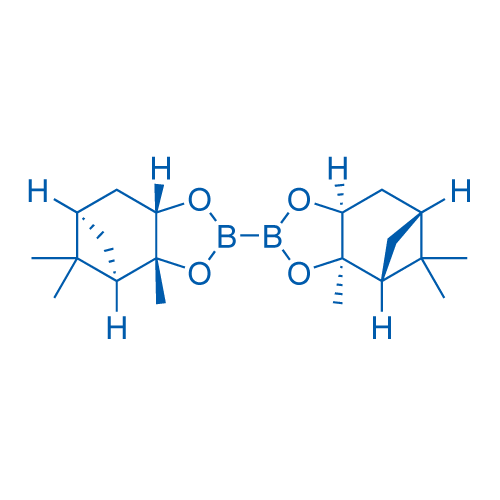
(3aR,3'aR,4R,4'R,6R,6'R,7aS,7'aS)-Dodecahydro-3a,3'a,5,5,5',5'-hexamethyl-2,2'-bi-4,6-methano-1,3,2-benzodioxaborole
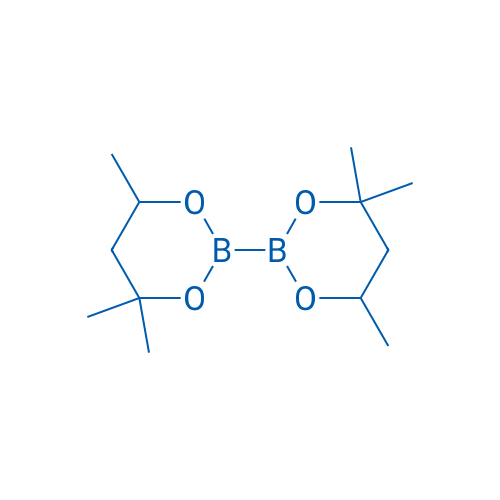
4,4,4',4',6,6'-Hexamethyl-2,2'-bi(1,3,2-dioxaborinane)
Diborons have been widely concerned in the field of organic chemistry due to their advantages such as good stability, easy storage, low cost and easy availability, especially in the borylation reaction catalyzed by transition metals.
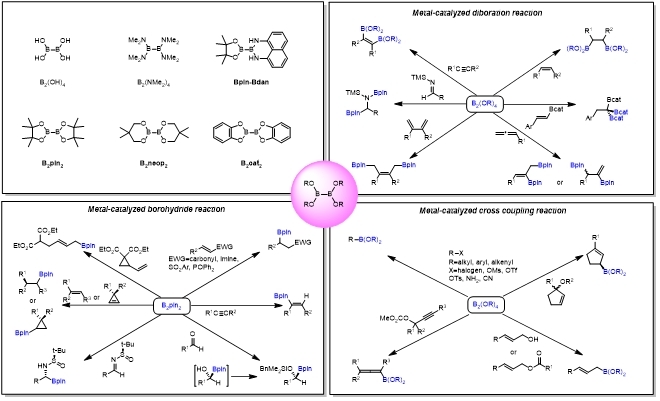
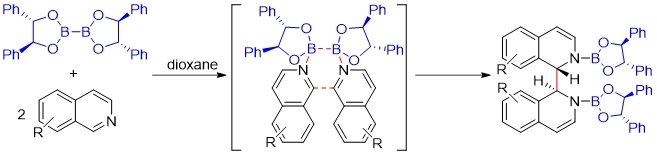
The application of diboron in organic synthesis has opened a new era with the report of metal-free diborylation of non-activated olefin. Varies mechanisms were proposed including heterolytic cleavage, hemolytic cleavage as well as concerted reaction. In 2017, Tang group reported a chiral diboron-templated highly diastereoselective reductive coupling of isoquinolines that provided expedited access to a series of chiral substituted bisisoquinolines. Mechanistic investigation suggests the reaction proceeds through a concerted [3,3]-sigmatropic rearrangement.
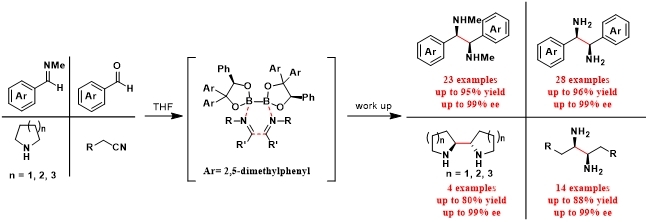
In 2017, Tang group reported a general, practical, and highly efficient method for asymmetric synthesis of a wide range of chiral vicinal diamines via reductive coupling of imines templated by chiral diboron.
References
[1]Suzuki, A. et al. J. Am. Chem. Soc. 1993, 115, 11018.
[2]Miyaura, N. et al. J. Org. Chem. 1995, 60, 7508.
[3]Molander, G. A. et al. J. Am. Chem. Soc. 2012, 134,16856.
[4]Morken, J. P. et al. J. Am. Chem. Soc. 2013, 135, 9252.
[5]Ito, H. et al. J. Am. Chem. Soc. 2015, 137, 420.
[6]Marder, T. B. et al. Chem. Rev. 2016, 116, 9091.
[7]Tang, W. et al. J. Am. Chem. Soc. 2017, 139, 9767.
[8]Tang, W. et al. J. Am. Chem. Soc. 2020, 142, 10337.