BLD Insights
Ready to get more kinds of Boronates from BLDpharm?
23 September 2022
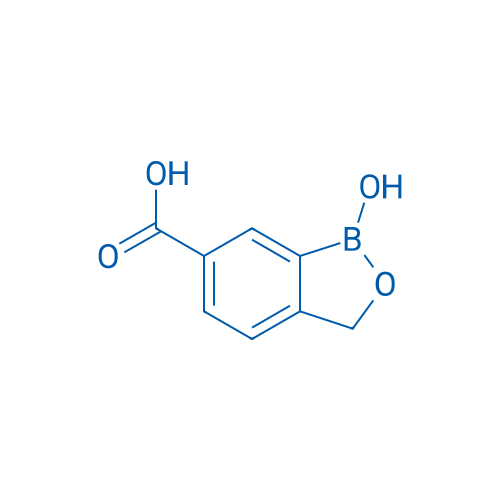
1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole-6-carboxylic acid
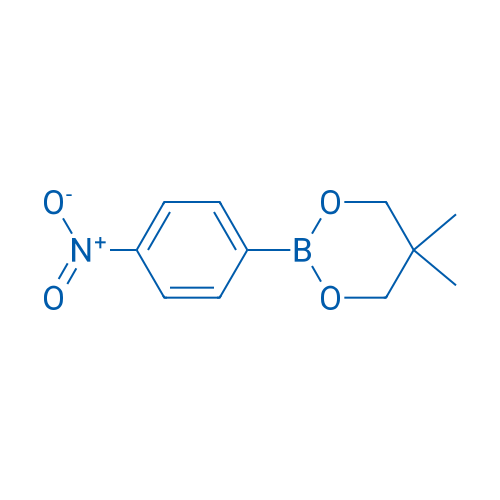
5,5-Dimethyl-2-(4-nitrophenyl)-1,3,2-dioxaborinane
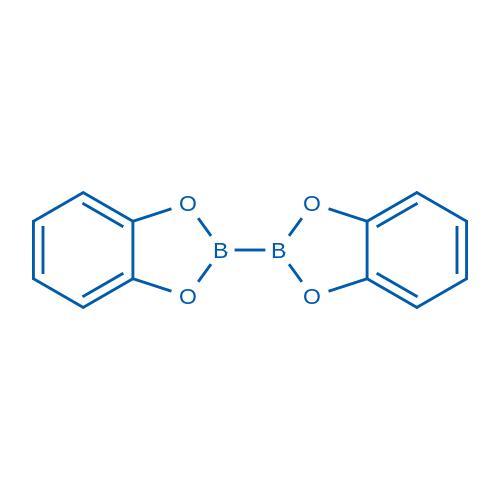
2,2'-Bibenzo[d][1,3,2]dioxaborole
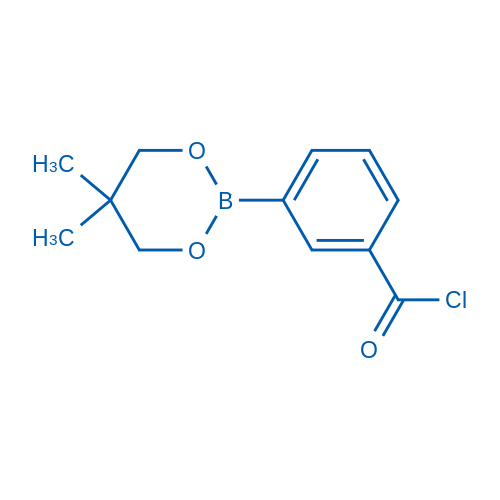
3-(5,5-Dimethyl-1,3,2-dioxaborinan-2-yl)benzoyl chloride
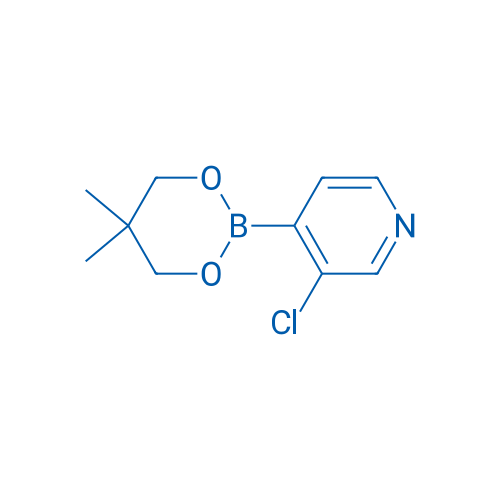
3-Chloro-4-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)pyridine
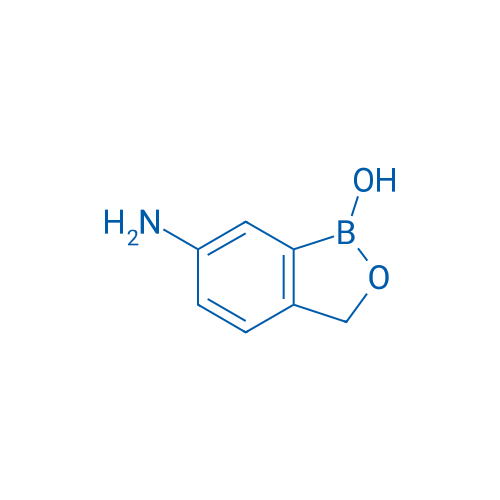
6-Aminobenzo[c][1,2]oxaborol-1(3H)-ol
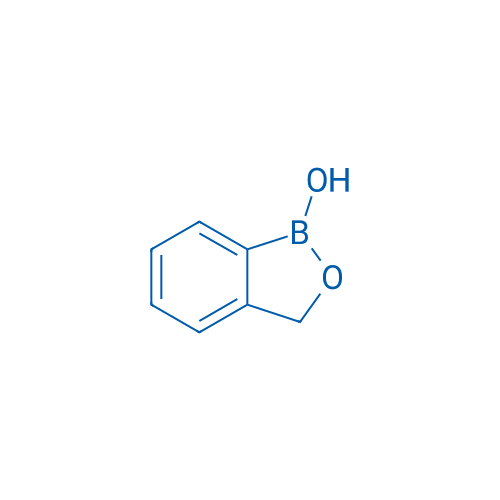
1-Hydroxy-2,1-benzoxaborolane

1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole-6-carboxylic acid

5,5-Dimethyl-2-(4-nitrophenyl)-1,3,2-dioxaborinane
Organobotonates including Bneops, Bcats, Bpinanes as well as [c][1,2]oxaborol-1(3H)-ols have a small number and a large variety compared to Bpins and boronic acids.
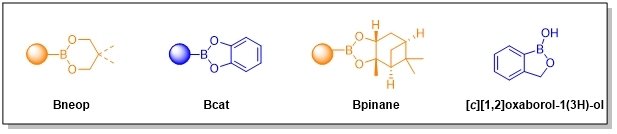
Special [c][1,2]oxaborol-1(3H)-ols such as Tavaborole and Crisaborole are BPC. Tavaborole is an antifungal agent that protects against trichophyton and is effective in treating onychomycosis while Tavaborole is a non-steroidal phosphodiesterase 4 (PDE-4) inhibitor. They can also be building blocks used to induce 2-benzyl alcohol group.
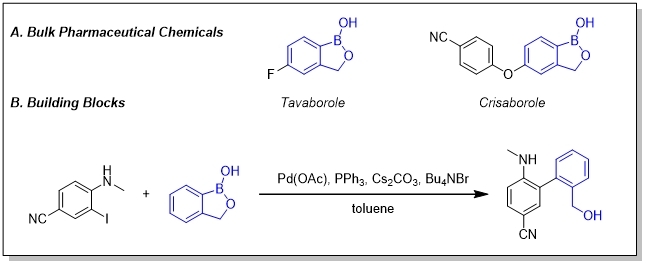
Bneops can be used in heteroaryl−heteroaryl SM cross-coupling reaction and C-H activation reaction. Also, due to the good water and fat solubility, they can be applicated for drug delivery. Bis[(-)-pinanediolato]diboron is a chiral reactant and both catalyst.
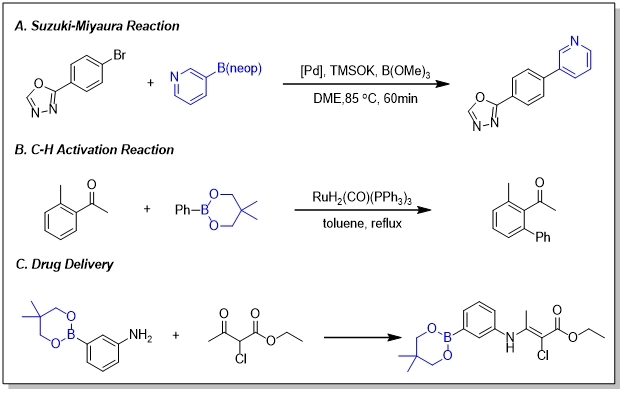
They have a wide range of applications in pharmaceutical chemistry and synthesis of natural products as well as methodology research.
References
[1]Martin D. Smith et al. J. Am. Chem. Soc. 2022, 144, 14790–14797.
[2]Scott E. Denmark et al. J. Am. Chem. Soc. 2021, 143, 13845–13853.
[3]Shinji Murai et al. J. Am. Chem. Soc. 2003, 125, 1698–1699.
[4]Huayue Wu et al. Chinese Chem. Lett. 2018, 29, 1795-1798.