BLD Insights
Application of Phosphine Oxide in Medicinal Chemistry
25 February 2022
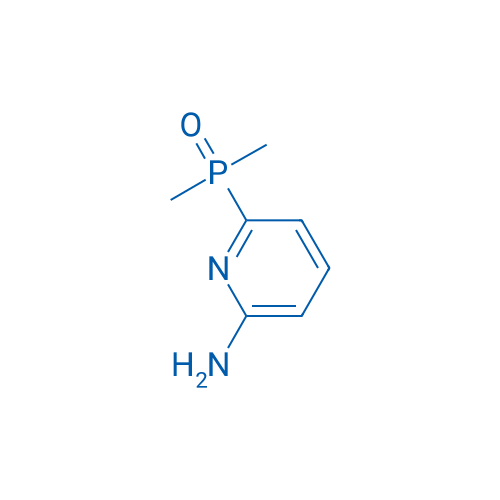
(6-Aminopyridin-2-yl)dimethylphosphine oxide
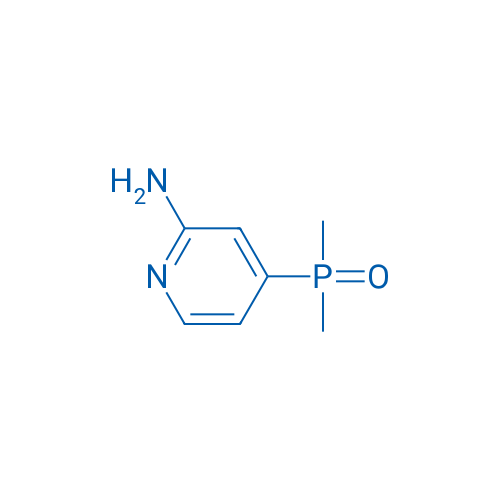
(2-Aminopyridin-4-yl)dimethylphosphine oxide
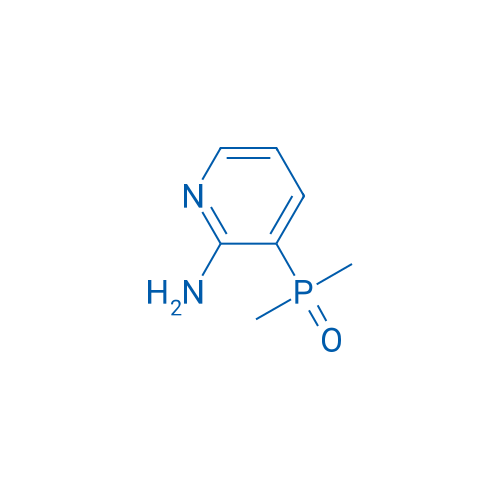
(2-Aminopyridin-3-yl)dimethylphosphine oxide
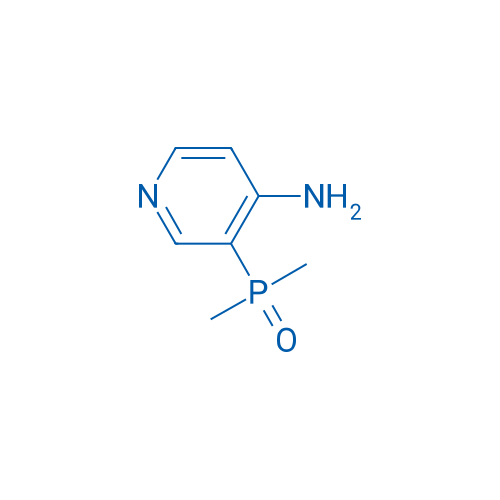
(4-Aminopyridin-3-yl)dimethylphosphine oxide
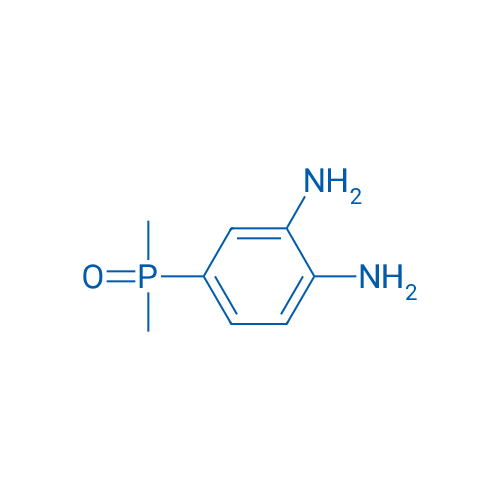
(3,4-Diaminophenyl)dimethylphosphine oxide
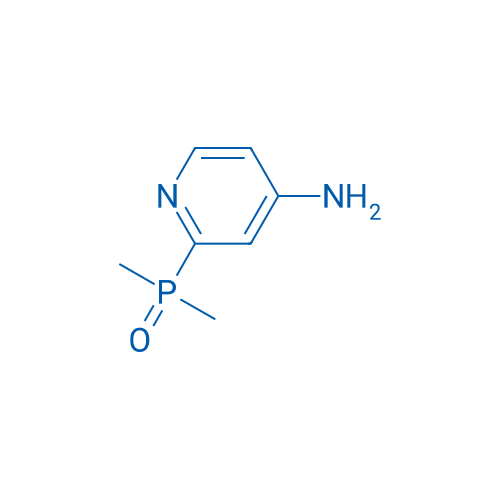
(4-Aminopyridin-2-yl)dimethylphosphine oxide
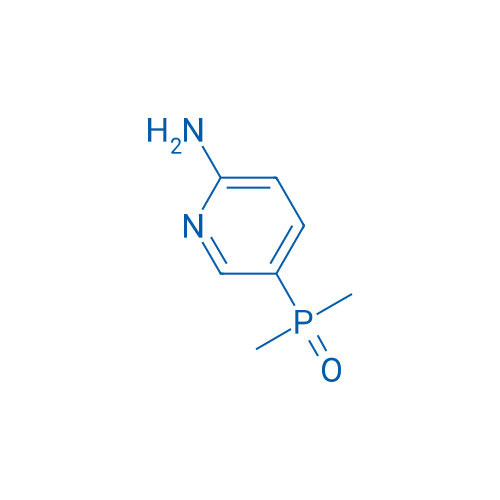
(6-Aminopyridin-3-yl)dimethylphosphine oxide
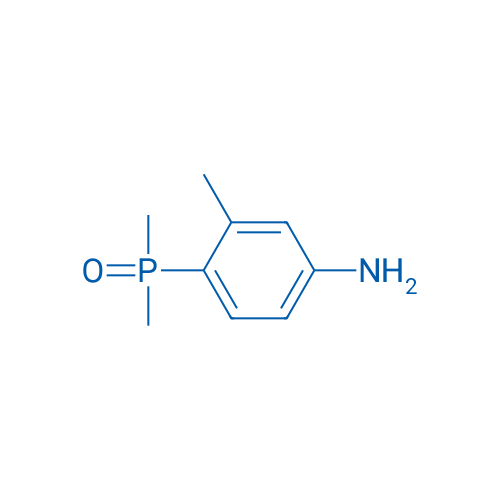
(4-Amino-2-methylphenyl)dimethylphosphine oxide
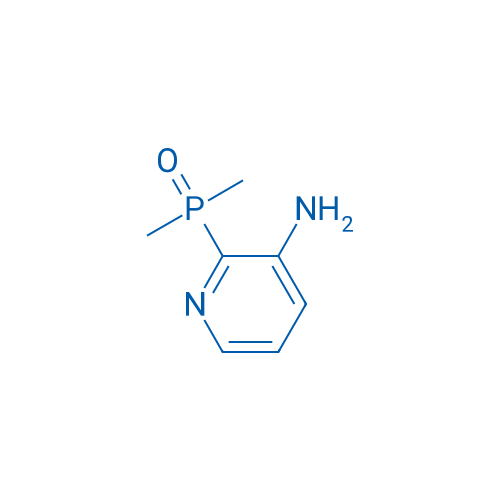
(3-Aminopyridin-2-yl)dimethylphosphine oxide
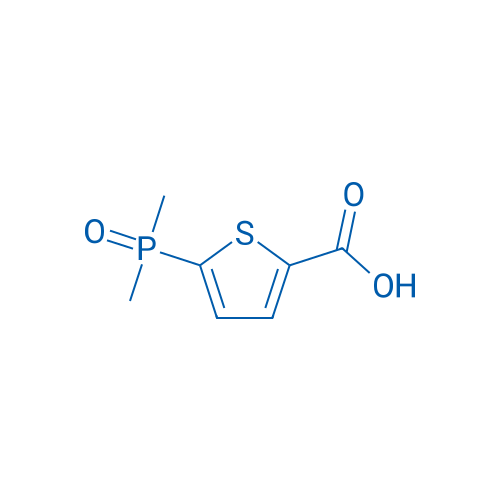
5-(Dimethylphosphoryl)thiophene-2-carboxylic acid

(6-Aminopyridin-2-yl)dimethylphosphine oxide

(2-Aminopyridin-4-yl)dimethylphosphine oxide

(2-Aminopyridin-3-yl)dimethylphosphine oxide

(4-Aminopyridin-3-yl)dimethylphosphine oxide
The majority of currently approved phosphorus-containing pharmaceuticals contain a phosphate, a phosphoramide, or a phosphonate group, while phosphine oxides are rare. [1] In 2017, brigatinib was approved by the Food and Drug Administration (FDA) as an anticancer drug.[2] This compound contained a fragment of phosphine oxide as shown in Figure 1. The application prospect of phosphine oxides in medicinal chemistry is gradually being valued by scientists.
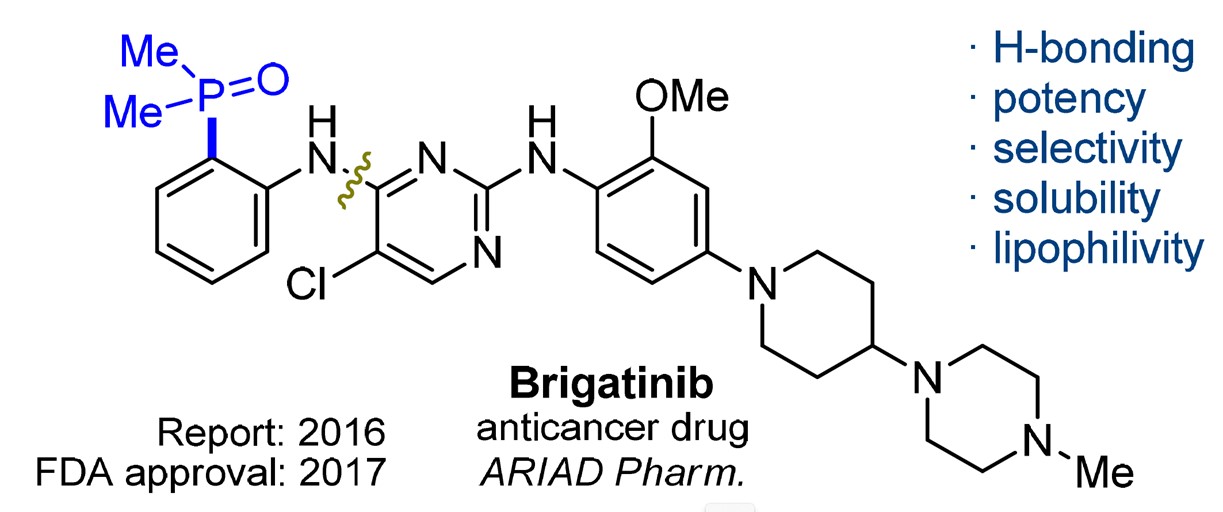
Figure 1. Structure of brigatinib
Advantages of in drug development
Phosphine oxides offer the unique structural feature of a very strong H-bond acceptor attached to a tetrahedral center with three potential vectors for derivatization as shown in Figure 2.[3]
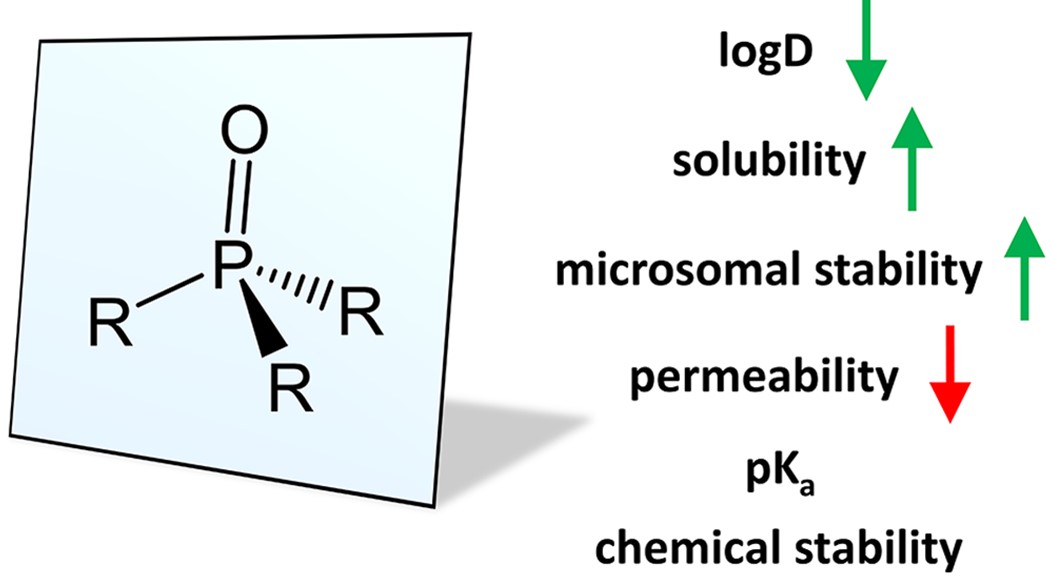
Figure2. Structure of phosphine oxides
Phosphine oxides possess sufficient chemical stability. They are more polar than some classical functional groups such as amides or sulfonamides, so the incorporation of the POMe2 substituent into organic compounds leads to a dramatic increase in solubility and decrease of lipophilicity. As shown in Figure 3, Prazosin (83) is a popular antihypertensive drug. However, its poor water solubility led to the development of a more soluble analog, terazosin (84). Recently, researchers found that the incorporation of the POMe2 substituent into Prazosin led to 85 which can significantly increase solubility and decrease lipophilicity while having similar biological profiles in vivo with Prazosin[4]. The increased polarity of the phosphine oxide analogs leads to improved metabolic stability, i.e., increased half-life in human liver microsomes. [5]
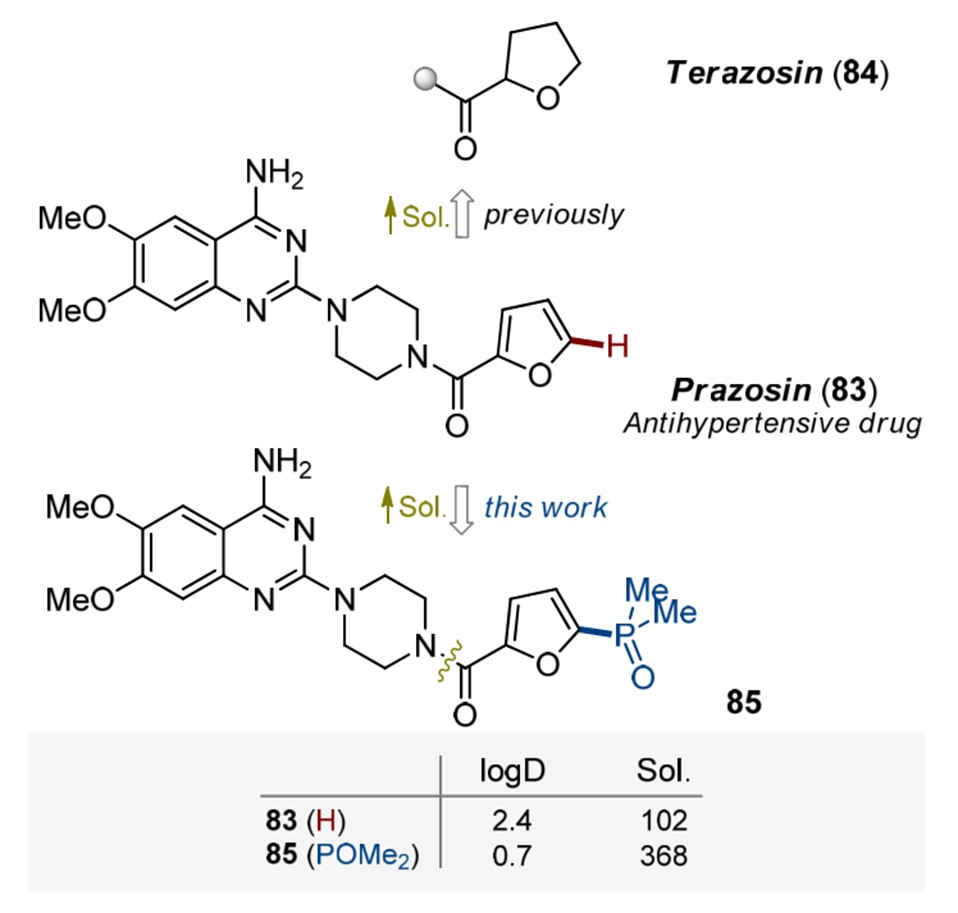
Figure 3. Incorporation of phosphine oxide into Prazosin
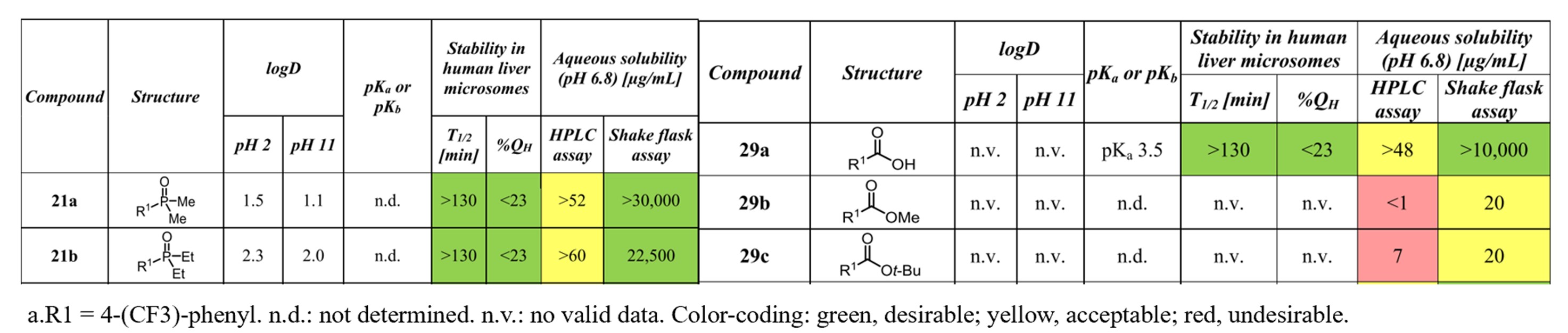
Figure 4. Introduction of phosphine oxide structure improves metabolic stability
Application case of phosphine oxide in drug development
Brigatinib, a second-generation anaplastic lymphoma kinase (ALK) inhibitor, has been approved for the treatment of ALK-positive non-small cell lung cancer (NSCLC) in many countries and regions such as the United States [6]. In 2016, ARIAD Pharmaceuticals based on the 2,4-diarylaminopyrimidine compounds as the core structure.[6] Then incorporated dimethylphosphine oxide (DMPO) into the 4' position of the 2'-methoxyaniline group and the ortho-substituted position on C4 aniline, respectively. As shown in Figure 5, the introduction of the phosphine oxide improved the activity and selectivity of the compound, which contributed to the successful development of brigatinib that provided a new treatment option for patients with non-small cell lung cancer.
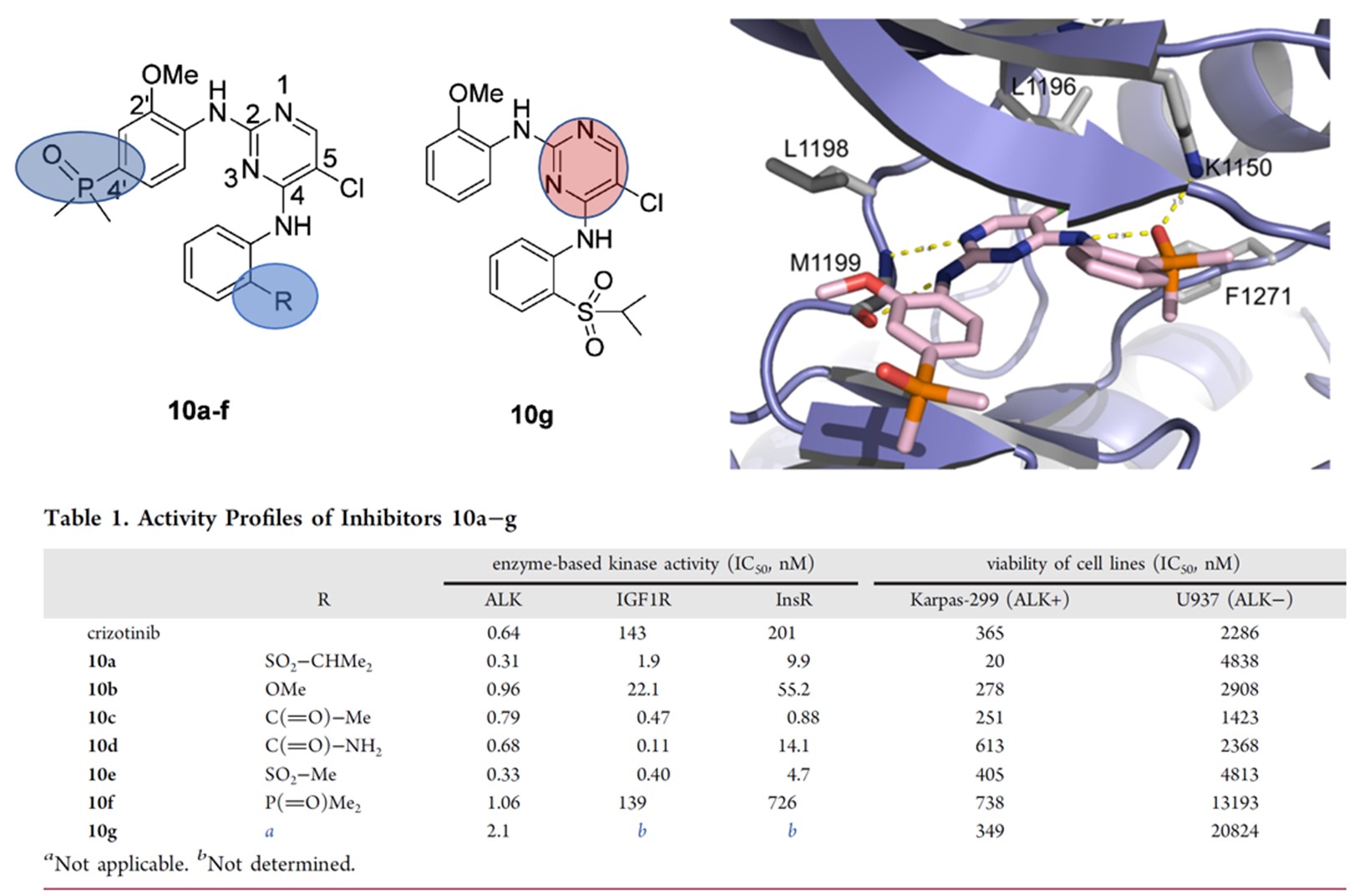
Figure 5. Discovery of brigatinib
Cyclin-dependent kinase 7 (CDK7) is a serine-threonine kinase that plays key roles in both transcription and regulation of the cell cycle. Due to its roles that are misregulated in cancer cells, CDK7 has emerged as a novel target in oncology. Recently Syros Pharmaceuticals reported a highly selective CDK7 noncovalent inhibitor SY-5609. [7] The researchers firstly designed lead compound 1, which exhibited highly potent inhibition of CDK7, however, its selectivity for CDKs was limited. The researchers further obtained compound 4 with increased potency and selectivity through computational simulation analysis. Then after the analysis of the crystal structures of compound 4 and CDK2, polar groups and heterocyclic structures were introduced into R2 and R3 of the indole benzene ring. The introduction of phosphine oxide led to compound 9 exhibiting excellent activity and selectivity. ADME properties show that the high polarity of the phosphine decreased the logD of compound 9, as shown in Figure 6. SY-5609 was obtained by further SAR, which not only effectively inhibited the proliferation of HCC70 cells but also has good oral bioavailability. The compound is currently being studied in a Phase 1 trial in patients with select solid tumors.
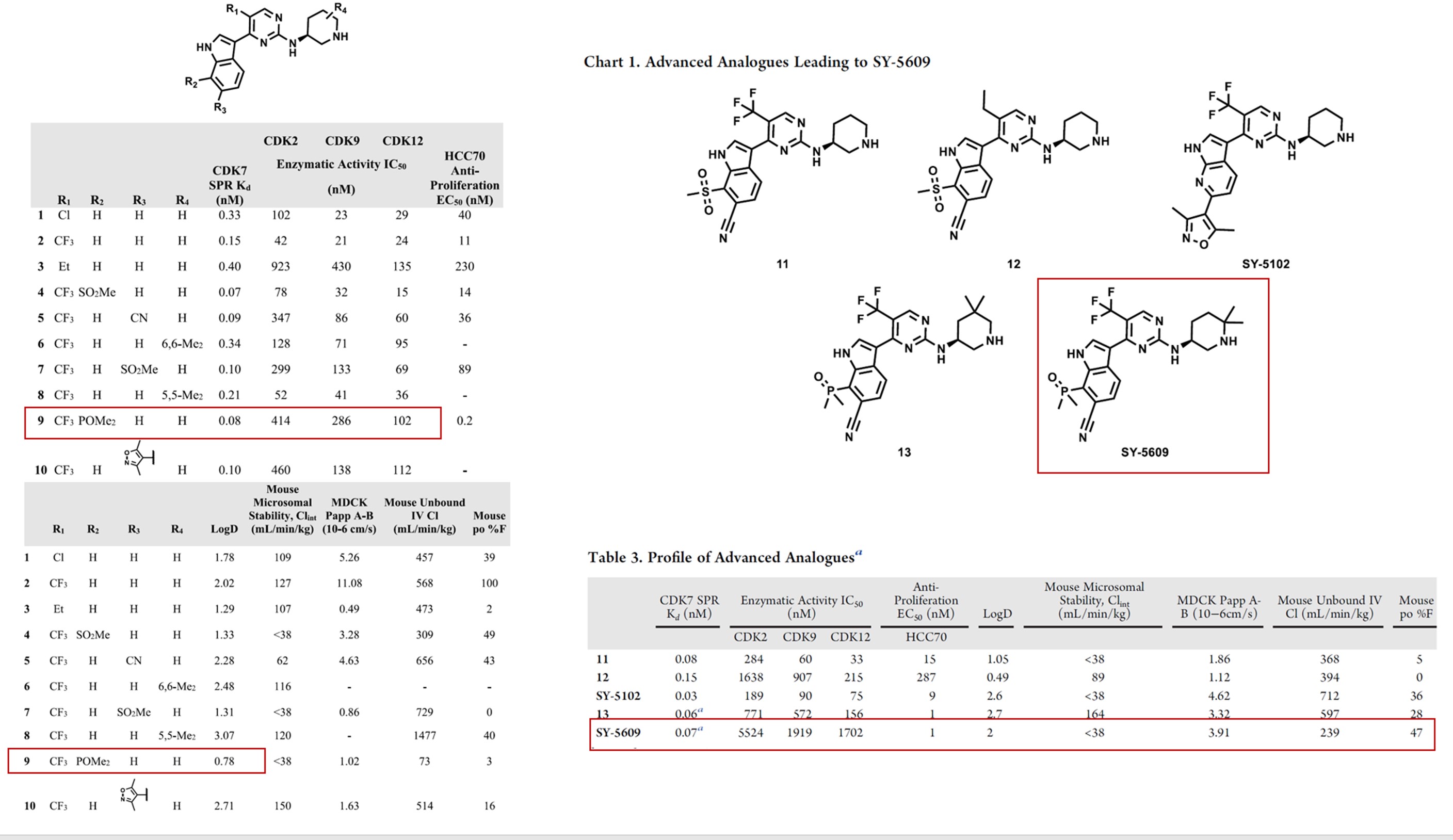
Figure 6. Structure and physicochemical properties of SY-5609
References
[1]Smith, B.R., C.M. Eastman, and J.T. Njardarson, Beyond C, H, O, and N! Analysis of the elemental composition of U.S. FDA approved drug architectures. J Med Chem, 2014. 57(23): p. 9764-73.
[2]https://www.fda.gov/drugs/resources-information-approved-drugs/brigatinib (accessed July 29, 2021).
[3]Laurence, C., et al., The pK(BHX) database: toward a better understanding of hydrogen-bond basicity for medicinal chemists. J Med Chem, 2009. 52(14): p. 4073-86.
[4]Stambirskyi, M.V., et al., Phosphine Oxides (-POMe2) for Medicinal Chemistry: Synthesis, Properties, and Applications. J Org Chem, 2021. 86(18): p. 12783-12801.
[5]Finkbeiner, P., J.P. Hehn, and C. Gnamm, Phosphine Oxides from a Medicinal Chemist's Perspective: Physicochemical and in Vitro Parameters Relevant for Drug Discovery. J Med Chem, 2020. 63(13): p. 7081-7107.
[6]Huang, W.S., et al., Discovery of Brigatinib (AP26113), a Phosphine Oxide-Containing, Potent, Orally Active Inhibitor of Anaplastic Lymphoma Kinase. J Med Chem, 2016. 59(10): p. 4948-64.
[7]Marineau, J.J., et al., Discovery of SY-5609: A Selective, Noncovalent Inhibitor of CDK7. J Med Chem, 2022. 65(2): p. 1458-1480.