BLD Insights
Salen Ligands and Co-Salen Complexes
14 February 2022
Salen ligands are prepared from primary diamines and salicylaldehydes. They are chelating ligands and could form stable metal-Salen complexes with various metal ions. The complexes play an important role in coordination chemistry and homogeneous catalysis. It has a wide range of application prospects in chiral catalysis, biomedicine and material science because of its excellent properties: (1) The synthesis of Salen ligands is convenient and the ligands can be recovered and reused after the reaction; (2) It has strong coordination ability and can form complexes with various metal ions; (3) It is suitable for a wide range of reactions, such as asymmetric catalytic oxidation of olefins, addition reaction, polymerization reaction, cyclopropanation reaction, Diels-Alder reaction, (photocatalytic) redox reaction, electrocatalytic reaction and kinetic resolution of epoxide hydrolysis. 1-7 (4) Metal-Salen complexes exhibit molecular magnetism, anti-tumor activity and optical properties in different research programs. 8-13
Development of Salen Ligands
ased on the research of porphyrin complexes, Jacobsen and Katsuki designed a similar ligand: Mn(III)-Salen complex in 1990-1991. The complex was used as a catalyst in asymmetric catalytic reactions (Jacobsen-Katsuki Epoxidation, Fig 1).14, 15
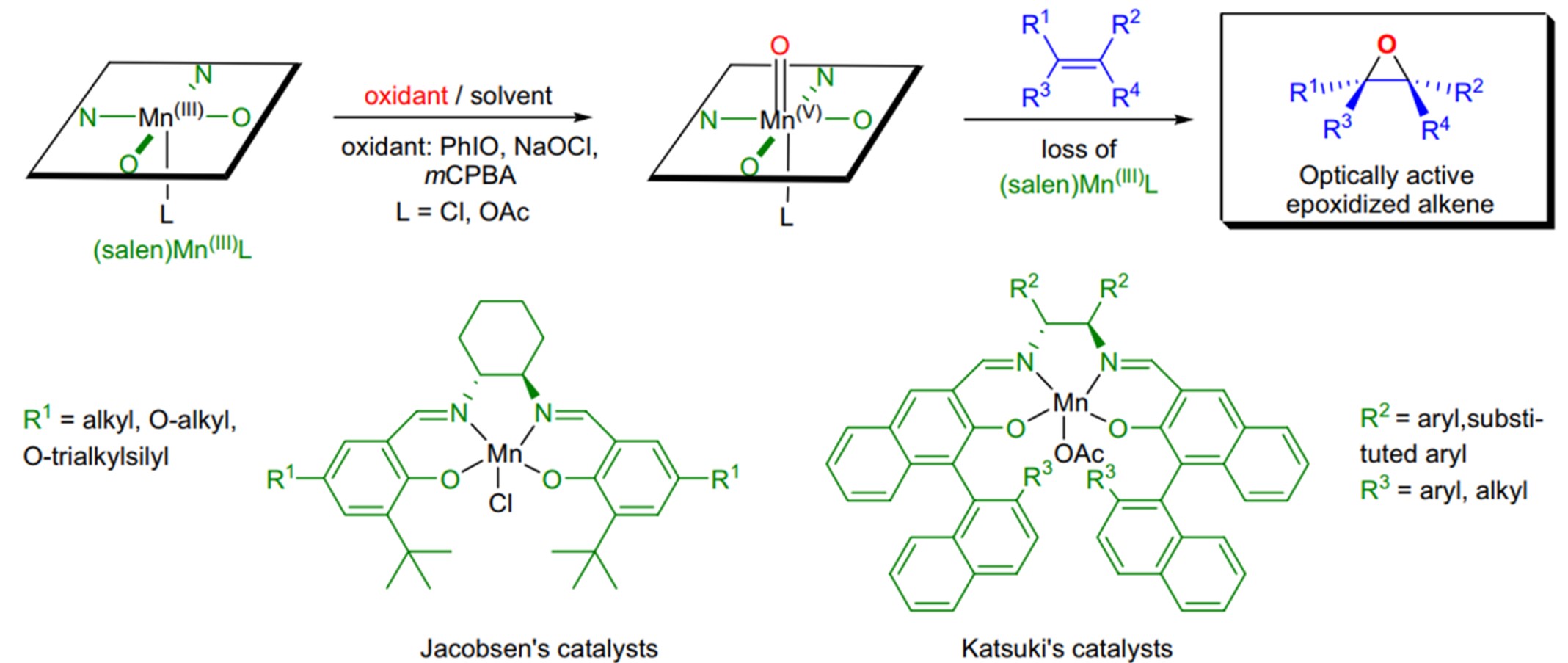
Fig 1: Jacobsen-Katsuki Epoxidation 16
Due to the four electron-donor sites (N/O atoms) in Salen ligands, the Metal-Salen complexes usually have a very high formation constant and can use different conformations (such as square planes, tetrahedron, square pyramid, and octahedron) to stabilize the spatial structures and control the catalytic performance of metal ions. In previous studies, various metal ions (including main-group metals and transition metals) have been used in the synthesis of Metal-Salen Complexes to generate a variety of complex catalysts for asymmetric catalysis, (photocatalytic) redox catalysis and other reactions.1, 17-21(Fig 2)
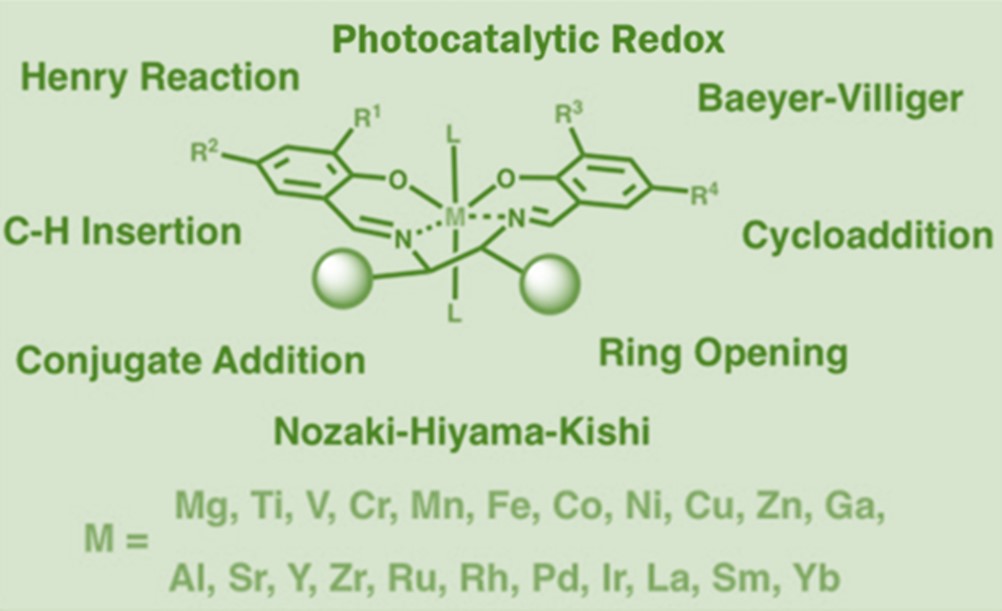
Fig 2: Metal-Salen complex catalysts2
Metal-Salen complexes with higher stereoselectivity and conversion have been continuously developed depending on the adjustment of (1) the connecting chain of diamine & (2) asymmetric substitution of salicylaldehyde derivatives.
Hotspot in Research & Application: Co(II/III)-Salen complex
Currently, catalyst systems mostly rely on precious metals such as ruthenium, rhodium, iridium, and platinum. However, the applications of these precious metal catalyst systems in industrial production are limited by their high toxicity, lack of reserves, and high price. Therefore, the development of catalytic systems based on cheap metals such as manganese, iron, cobalt, and nickel has become a research hotspot in recent years. The environmentally friendly and inexpensive transition metal cobalt has attracted extensive attention due to its high catalytic activity and stereoselectivity. Hence, Co-Salen complexes are constantly being developed and widely used. They are usually used in oxidation, polymerization and asymmetric catalysis. 13, 18, 22-26 (Fig 3A-C)
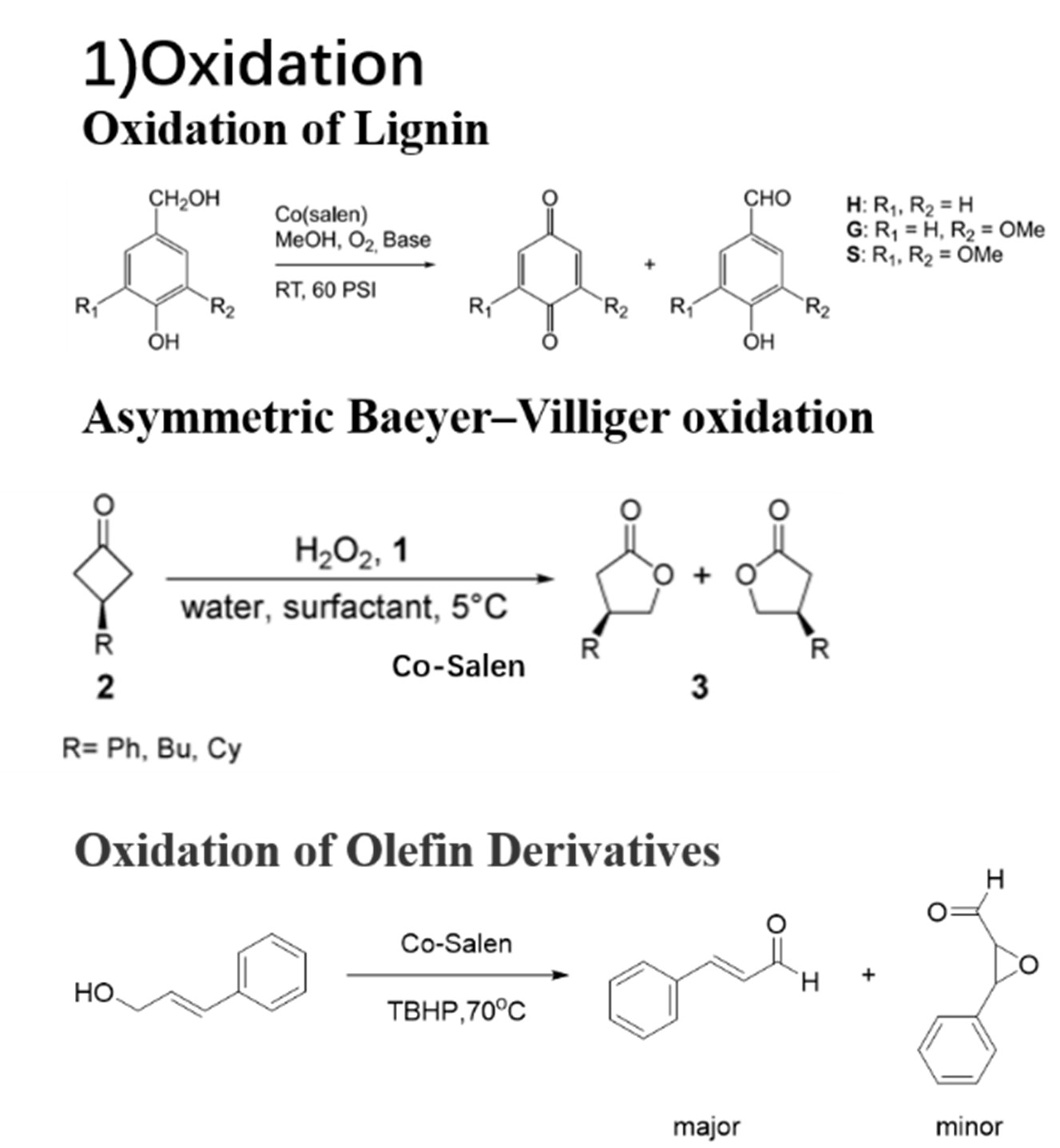
Fig 3A: Oxidations catalyzed by Co-Salen complexes
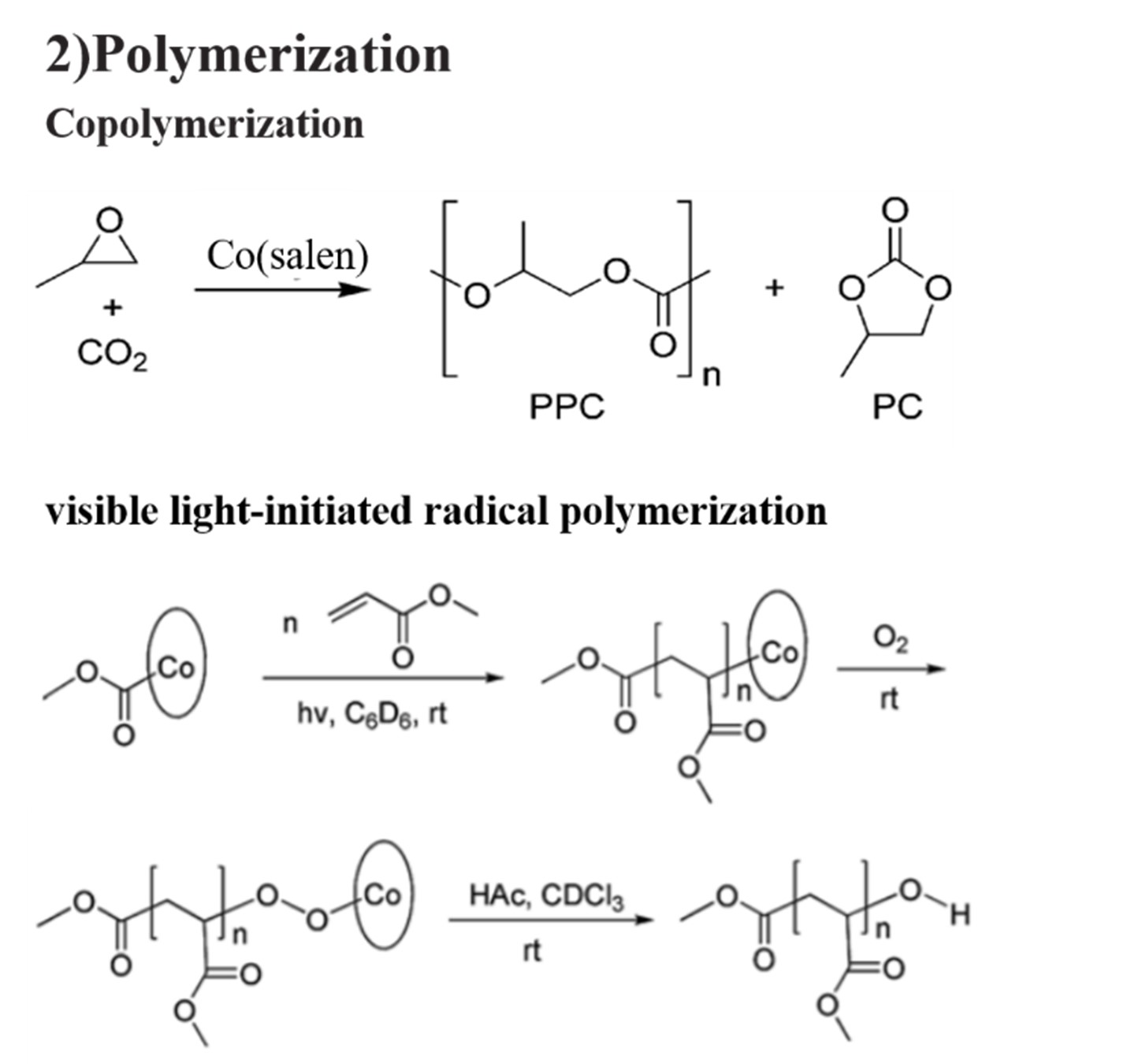
Fig 3B: Polymerizations catalyzed by Co-Salen complexes
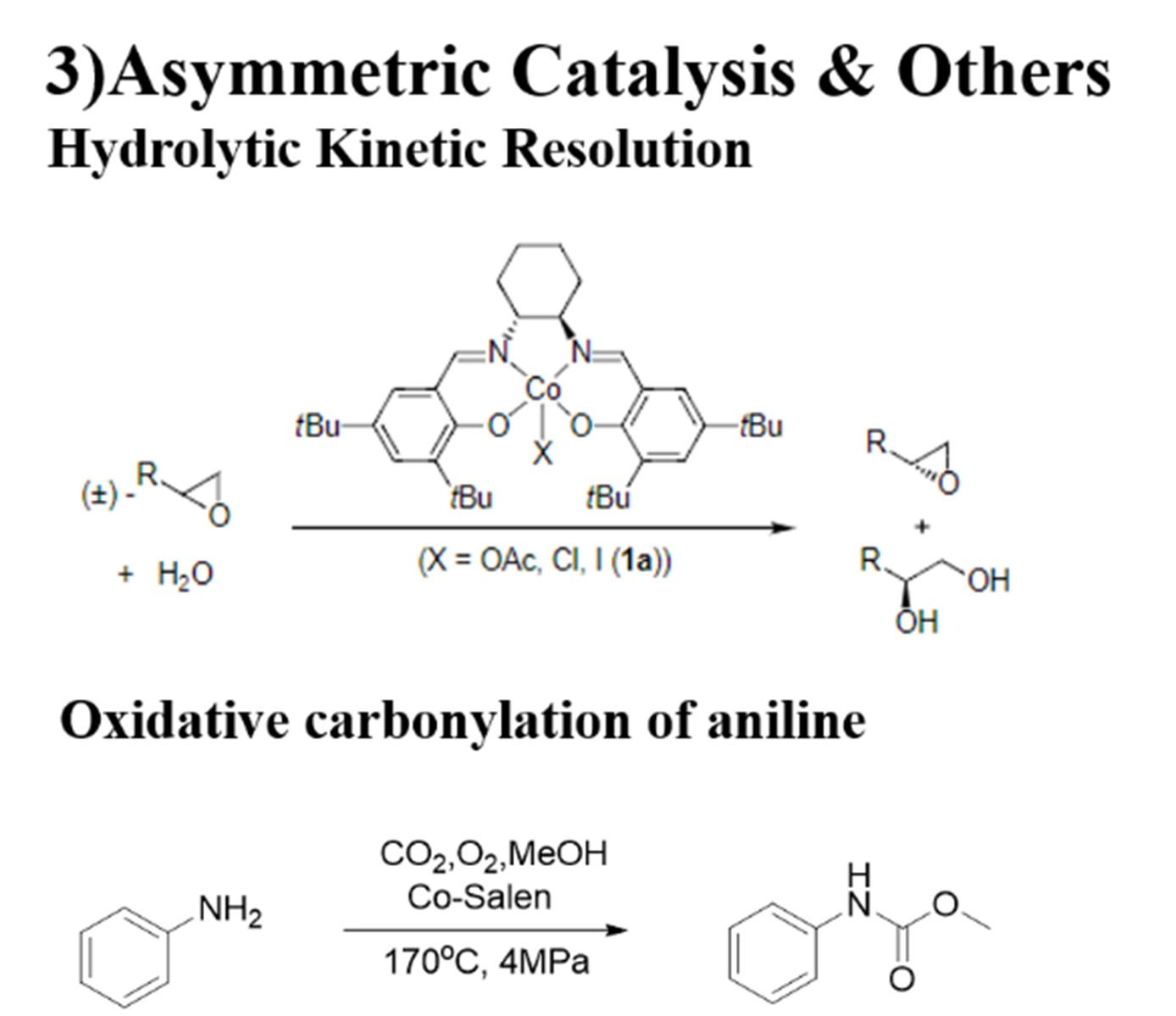
Fig 3C: asymmetric catalysis & other applications of Metal-Salen complexes
The research projects on Co-Salen complexes in biosensors, material applications (MOF/magnetic materials), tumor treatment and other fields are also being carried out accordingly. (Fig 4) 13, 22, 27-30
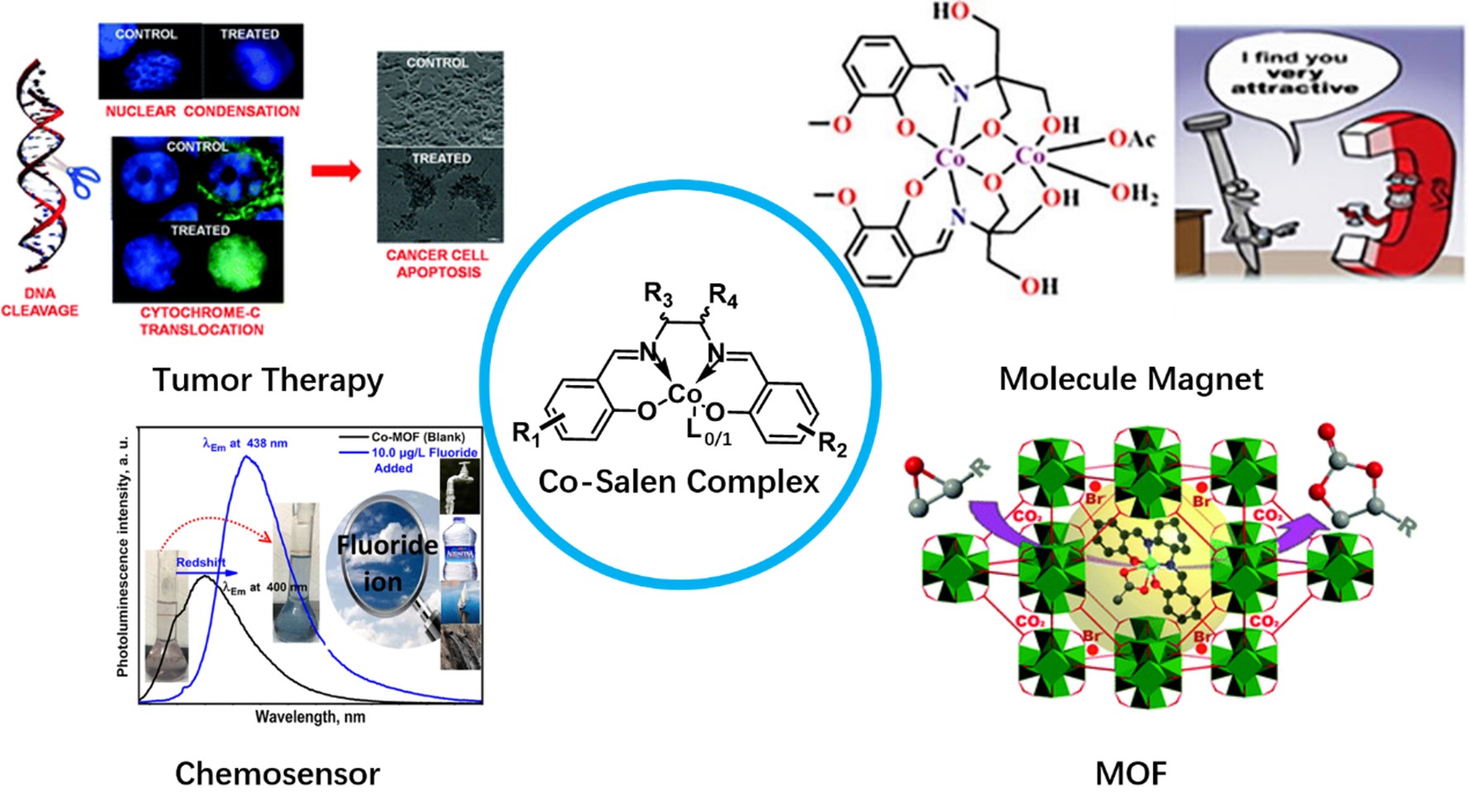
Fig 4: Applications of Co-Salen complexes
References
[1]Zhang, R.; Klaine, S.; Alcantar, C.; Bratcher, F., Visible light generation of high-valent metal-oxo intermediates and mechanistic insights into catalytic oxidations. Journal of Inorganic Biochemistry 2020, 212, 111246.
[2]Shaw, S.; White, J. D., Asymmetric Catalysis Using Chiral Salen–Metal Complexes: Recent Advances. Chemical Reviews 2019, 119, 9381-9426.
[3]Osten, K. M.; Mehrkhodavandi, P., Indium Catalysts for Ring Opening Polymerization: Exploring the Importance of Catalyst Aggregation. Accounts of Chemical Research 2017, 50, 2861-2869.
[4]Azam, M.; Kumar, U.; Olowoyo, J. O.; Al-Resayes, S. I.; Trzesowska-Kruszynska, A.; Kruszynski, R.; Islam, M. S.; Khan, M. R.; Adil, S. F.; Siddiqui, M. R.; Al-Harthi, F. A.; Alinzi, A. K.; Wabaidur, S. M.; Siddiqui, M. R.; Shaik, M. R.; Jain, S. L.; Farkhondehfal, M. A.; Hernàndez, S., Dinuclear uranium(vi) salen coordination compound: an efficient visible-light-active catalyst for selective reduction of CO2 to methanol. Dalton Transactions 2020, 49, 17243-17251.
[5]North, M.; Quek, S. C. Z.; Pridmore, N. E.; Whitwood, A. C.; Wu, X., Aluminum(salen) Complexes as Catalysts for the Kinetic Resolution of Terminal Epoxides via CO2 Coupling. ACS Catalysis 2015, 5, 3398-3402.
[6]Jia, F.; Chen, X.; Zheng, Y.; Qin, Y.; Tao, Y.; Wang, X., One-pot atom-efficient synthesis of bio-renewable polyesters and cyclic carbonates through tandem catalysis. Chemical Communications 2015, 51, 8504-8507.
[7]Singh, S.; Phukan, B.; Mukherjee, C.; Verma, A., Salen ligand complexes as electrocatalysts for direct electrochemical reduction of gaseous carbon dioxide to value added products. RSC Advances 2015, 5, 3581-3589.
[8]Consiglio, G.; Oliveri, I. P.; Failla, S.; Di Bella, S., On the Aggregation and Sensing Properties of Zinc(II) Schiff-Base Complexes of Salen-Type Ligands. Molecules 2019, 24.
[9]Rouco, L.; González-Noya, A. M.; Pedrido, R.; Maneiro, M., Pursuing the Elixir of Life: In Vivo Antioxidative Effects of Manganosalen Complexes. Antioxidants 2020, 9.
[10]A. Rosenthal, R.; Fish, B.; P. Hill, R.; D. Huffman, K.; Lazarova, Z.; Mahmood, J.; Medhora, M.; Molthen, R.; E. Moulder, J.; T. Sonis, S.; J. Tofilon, P.; R. Doctrow, S., Salen Mn Complexes Mitigate Radiation Injury in Normal Tissues. Anti-Cancer Agents in Medicinal Chemistry 2011, 11, 359-372.
[11]Liu, Y.; Xi, X.; Ye, C.; Gong, T.; Yang, Z.; Cui, Y., Chiral Metal–Organic Frameworks Bearing Free Carboxylic Acids for Organocatalyst Encapsulation. Angewandte Chemie International Edition 2014, 53, 13821-13825.
[12]Zhu, W.; Du, L.; Li, W.; Zuo, J.; Shan, J., The salen based chemosensors for highly selective recognition of Zn2+ ion. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2018, 203, 501-509.
[13]Ali, A.; Kamra, M.; Bhan, A.; Mandal, S. S.; Bhattacharya, S., New Fe(iii) and Co(ii) salen complexes with pendant distamycins: selective targeting of cancer cells by DNA damage and mitochondrial pathways. Dalton Transactions 2016, 45, 9345-9353.
[14]Zhang, W.; Loebach, J. L.; Wilson, S. R.; Jacobsen, E. N., Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes. Journal of the American Chemical Society 1990, 112, 2801-2803.
[15]Jacobsen, E. N.; Zhang, W.; Muci, A. R.; Ecker, J. R.; Deng, L., Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane. Journal of the American Chemical Society 1991, 113, 7063-7064.
[16]Holman, R. W., Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms (Kürti, László; Czakó, Barbara). Journal of Chemical Education 2005, 82, 1780.
[17]Wei, P.; Atwood, D. A., Bimetallic Borate Derivatives of the Salen Ligands. Inorganic Chemistry 1997, 36, 4060-4065.
[18]Cooper, C. J.; Alam, S.; Nziko, V. d. P. N.; Johnston, R. C.; Ivanov, A. S.; Mou, Z.; Turpin, D. B.; Rudie, A. W.; Elder, T. J.; Bozell, J. J.; Parks, J. M., Co(salen)-Catalyzed Oxidation of Lignin Models to Form Benzoquinones and Benzaldehydes: A Computational and Experimental Study. ACS Sustainable Chemistry & Engineering 2020, 8, 7225-7234.
[19]Cohen, C. T.; Chu, T.; Coates, G. W., Cobalt Catalysts for the Alternating Copolymerization of Propylene Oxide and Carbon Dioxide: Combining High Activity and Selectivity. Journal of the American Chemical Society 2005, 127, 10869-10878.
[20]Jakhar, A.; Sadhukhan, A.; Khan, N.-u. H.; Saravanan, S.; Kureshy, R. I.; Abdi, S. H. R.; Bajaj, H. C., Asymmetric Hydrocyanation of Nitroolefins Catalyzed by an Aluminum(III) Salen Complex. ChemCatChem 2014, 6, 2656-2661.
[21]Gualandi, A.; Calogero, F.; Potenti, S.; Cozzi, P. G., Al(Salen) Metal Complexes in Stereoselective Catalysis. Molecules 2019, 24.
[22]Zhao, Y.; Yu, M.; Zhang, S.; Wu, Z.; Liu, Y.; Peng, C.-H.; Fu, X., A well-defined, versatile photoinitiator (salen)Co–CO2CH3 for visible light-initiated living/controlled radical polymerization. Chemical Science 2015, 6, 2979-2988.
[23]Annis, D. A.; Jacobsen, E. N., Polymer-Supported Chiral Co(Salen) Complexes: Synthetic Applications and Mechanistic Investigations in the Hydrolytic Kinetic Resolution of Terminal Epoxides. Journal of the American Chemical Society 1999, 121, 4147-4154.
[24]Breinbauer, R.; Jacobsen, E. N., Cooperative Asymmetric Catalysis with Dendrimeric [Co(salen)] Complexes. Angewandte Chemie International Edition 2000, 39, 3604-3607.
[25]Bianchini, G.; Cavarzan, A.; Scarso, A.; Strukul, G., Asymmetric Baeyer–Villiger oxidation with Co(Salen) and H2O2 in water: striking supramolecular micelles effect on catalysis. Green Chemistry 2009, 11, 1517-1520.
[26]Dhinagaran, G.; Prashanna Suvaitha, S.; Muthukumaran, M.; Venkatachalam, K., Chemoselective Catalytic Oxidation of Olefin Derivatives with Co–Salen Immobilized SBA-15. Catalysis Letters 2021, 151, 1361-1375.
[27]Alhaddad, M.; El-Sheikh, S. M., Selective and Fast Detection of Fluoride-Contaminated Water Based on a Novel Salen-Co-MOF Chemosensor. ACS Omega 2021, 6, 15182-15191.
[28]Medina, S.; Henderson, A. S.; Bower, J. F.; Galan, M. C., Stereoselective synthesis of glycosides using (salen)Co catalysts as promoters. Chemical Communications 2015, 51, 8939-8941.
[29]Liu, T.-T.; Liang, J.; Xu, R.; Huang, Y.-B.; Cao, R., Salen-Co(iii) insertion in multivariate cationic metal–organic frameworks for the enhanced cycloaddition reaction of carbon dioxide. Chemical Communications 2019, 55, 4063-4066.
[30]Banerjee, A.; Chattopadhyay, S., Synthesis and characterization of mixed valence cobalt(III)/cobalt(II) complexes with N,O-donor Schiff base ligands. Polyhedron 2019, 159, 1-11.