BLD Insights
The Utilization of Spirocyclic Scaffolds in Medicinal Chemistry
30 November 2021
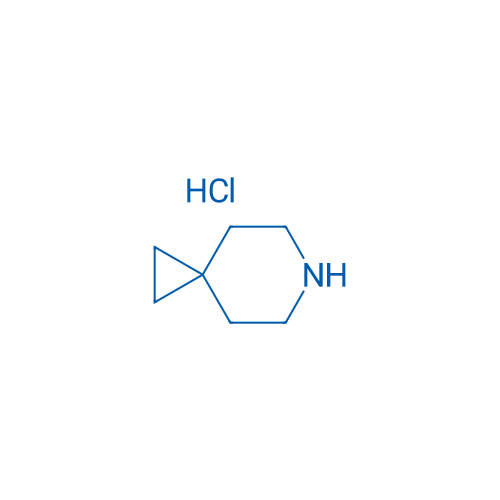
6-Azaspiro[2.5]octane hydrochloride
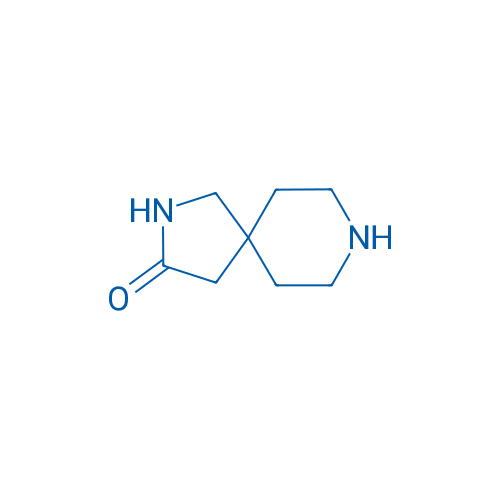
2,8-Diazaspiro[4.5]decan-3-one
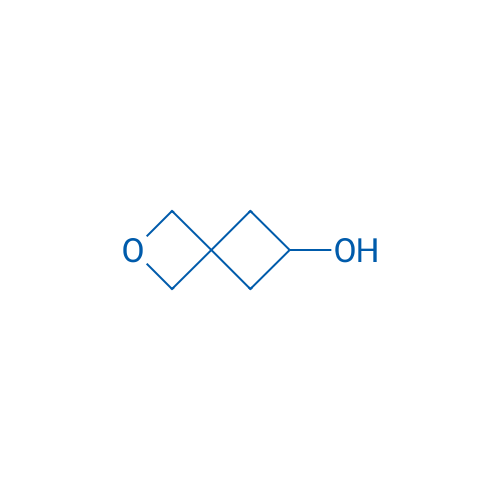
2-Oxaspiro[3.3]heptan-6-ol
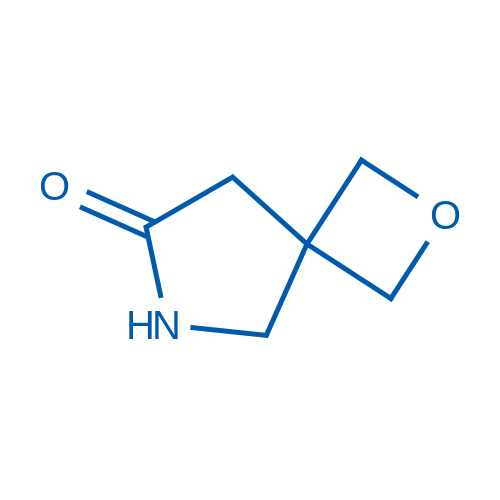
2-Oxa-6-azaspiro[3.4]octan-7-one
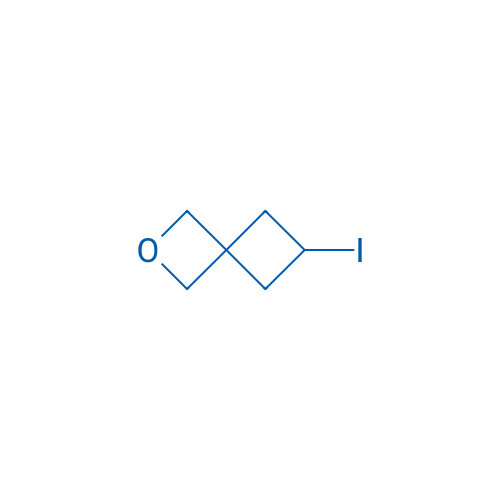
6-Iodo-2-oxaspiro[3.3]heptane
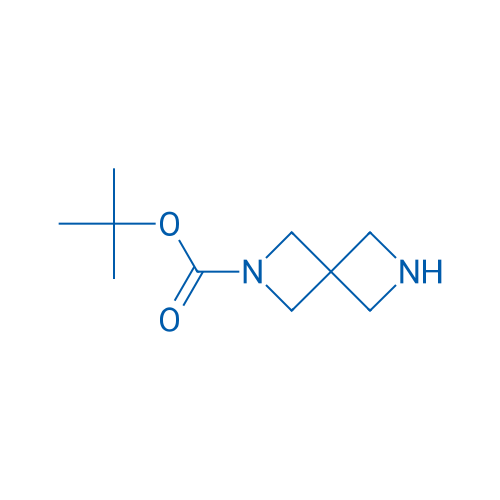
tert-Butyl 2,6-diazaspiro[3.3]heptane-2-carboxylate
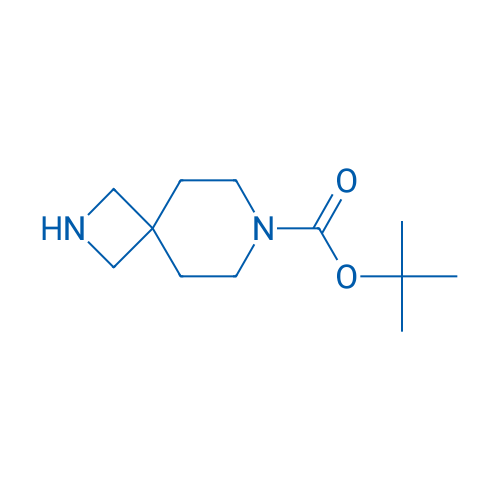
tert-Butyl 2,7-diazaspiro[3.5]nonane-7-carboxylate
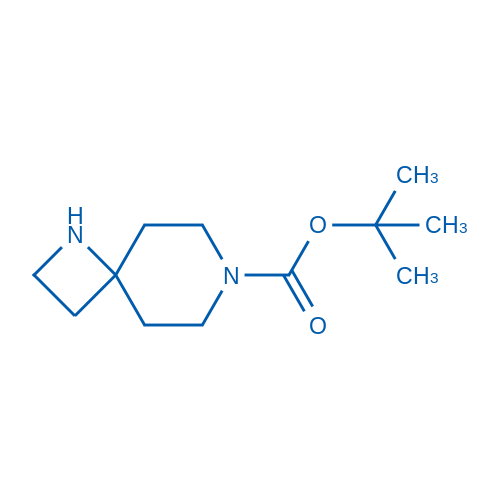
tert-Butyl 1,7-diazaspiro[3.5]nonane-7-carboxylate
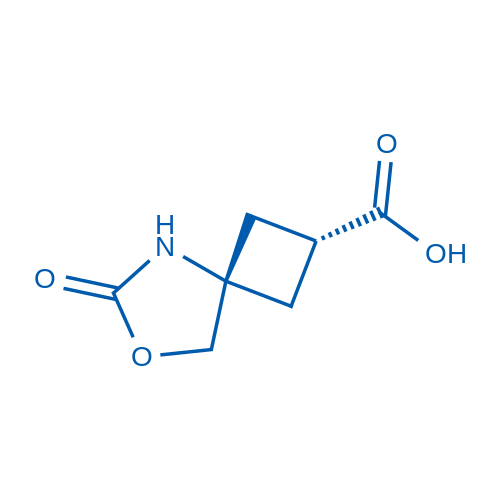
cis-6-Oxo-7-oxa-5-azaspiro[3.4]octane-2-carboxylic acid
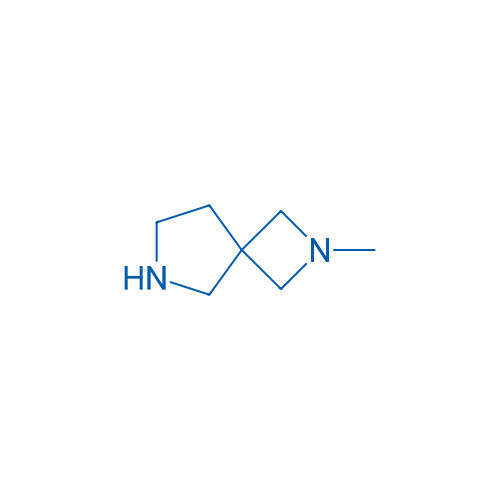
2-Methyl-2,6-diazaspiro[3.4]octane

6-Azaspiro[2.5]octane hydrochloride

2,8-Diazaspiro[4.5]decan-3-one

2-Oxaspiro[3.3]heptan-6-ol

2-Oxa-6-azaspiro[3.4]octan-7-one
Spirocycles are ring systems in which two rings are fused by a single atom (Zheng and Tice, 2016). Spirocyclic scaffolds are widely used in medicinal chemistry, which have been paid increasing interest in by the development of modern drugs. Currently, a wide range of spirocyclic scaffolds can be recognized in drug candidates and approved drugs (Figure 1) (Hiesinger et al., 2021).
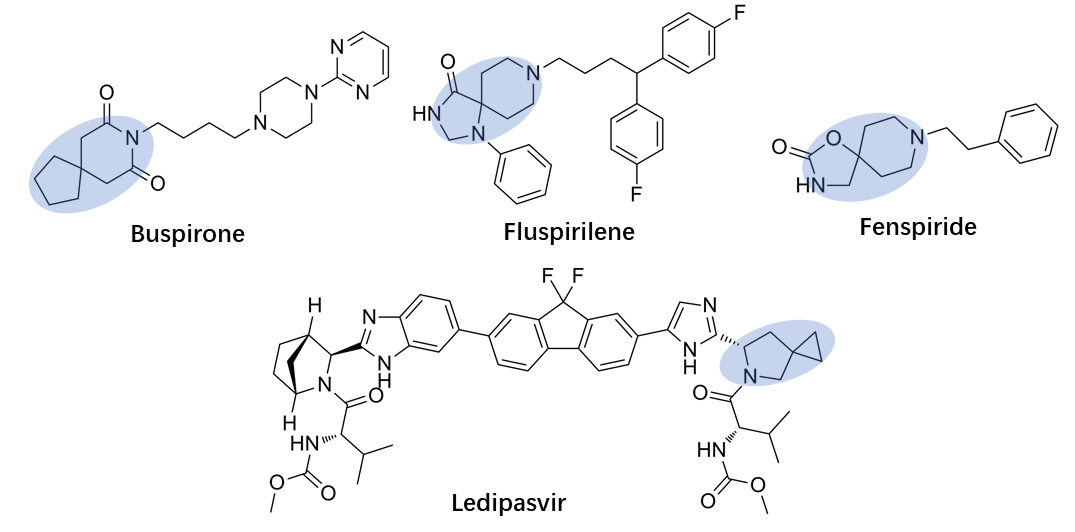
Figure 1. Approved Drugs Containing a Spirocycle
Advantages of spirocyclic scaffolds
The main advantage that spirocycles offer as core structures is their inherent three-dimensional nature and ability to modulate molecule physicochemical properties. (Zheng and Tice, 2016).
The quarternary carbon structures of spirocycles lead to higher Fsp3, where Fsp3= (number of sp3 hybridized carbons/total carbon count). It is used to measure the complexity of the compound. A higher fraction of sp3 has an increased probability to succeed in compound translation to the clinic, and it may be mainly due to out-of-plane substituents, which is conducive to adjusting the molecular shape and increasing receptor/ligand complementarity (Figure 2) (Lovering et al., 2009). Spiro-containing systems increase the Fsp3 value of the molecule, modulate physicochemical properties such as water solubility, log P, and metabolic stability, and are more likely to be successfully developed as drugs. For example, Johansson et al. developed melanin concentrating hormone receptor 1 (MCHr1) antagonists. During the optimization process, exchanging the morpholine to diverse azaspiro cycles lowered log D values, and improved the selectivity between MCHr1 and hERG and the metabolic stability (Figure 3) (Johansson et al., 2016).
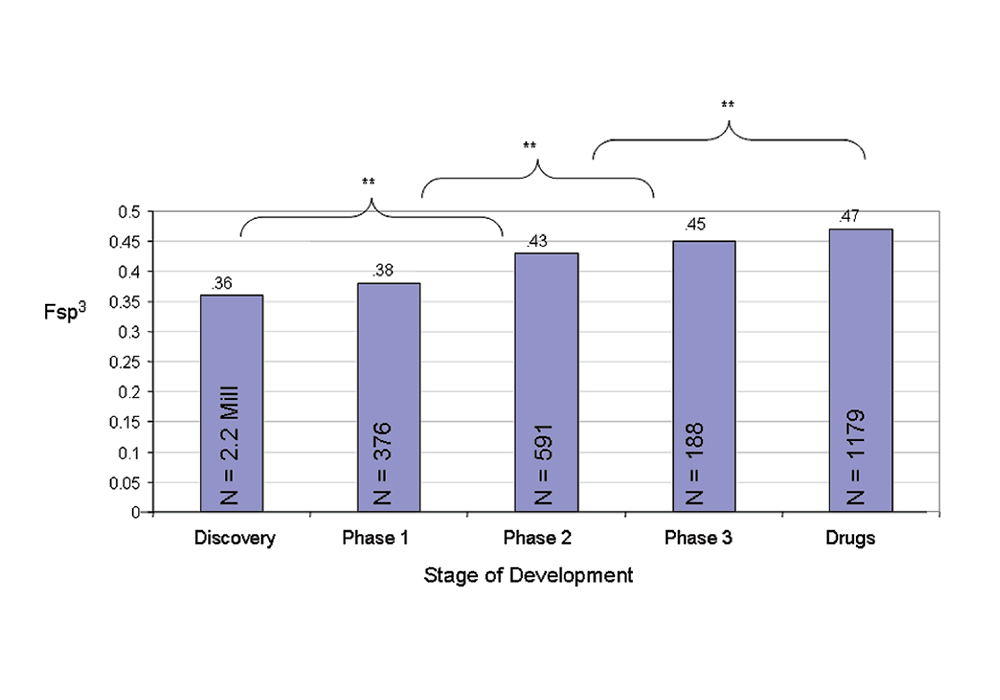
Figure 2. Mean Fsp3 for compounds in different stages of development
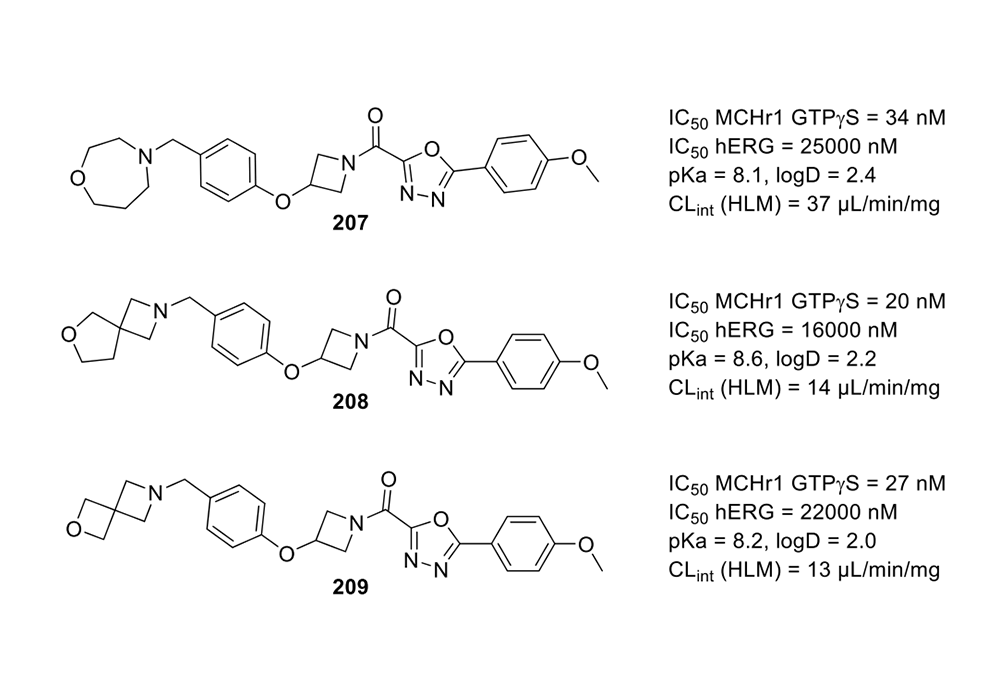
Figure 3. Spirocyclic MCHr1 antagonists
Because of the inherent three-dimensional nature of spirocycles, introducing a rigid spirocyclic scaffold could replace rotatable bonds and maintain the favored orientation of the functional group to improve potency. For example, during optimization of protein tyrosine phosphatase 2 (SHP2) inhibitors, the researchers analyzed X-ray structure and introduced spirocyclic scaffolds to maintain the orientation of the primary amine group and keep the three directed H-bond interactions. All spirocyclic variants exhibited comparable potency on SHP2 protein inhibitory and the cellular efficacy was improved (Figure 4)(Bagdanoff et al., 2019; Sarver et al., 2019).
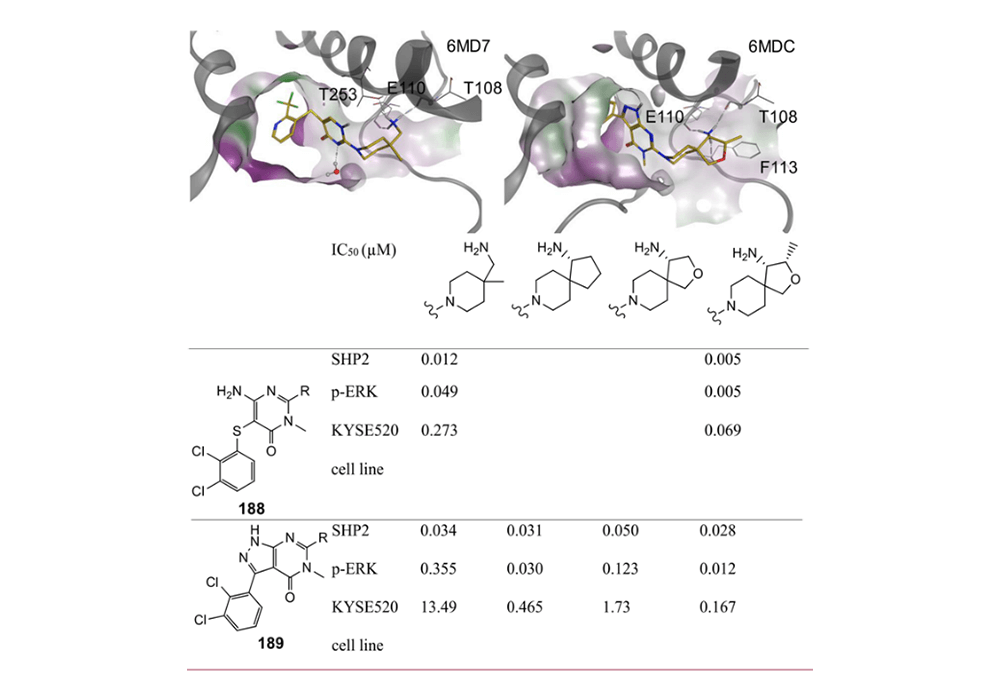
Figure 4. Structure-Based Introduction of a Spirocycle into Two Series of SHP2 Inhibitors
Spirocyclic scaffolds can also influence selectivity. For example, the piperazine of poly (ADP-ribose) polymerase (PARP) inhibitor, Olaparib, was substituted with diazaspiro [3.3] heptane to obtain 197. Although the spirocyclic analogue showed a 2-fold reduction in potency, the selectivity for RARP-1 against other members of the PARP family increased significantly, which came along with reduced DNA damage and cytotoxicity (Figure 5)(Reilly et al., 2018).
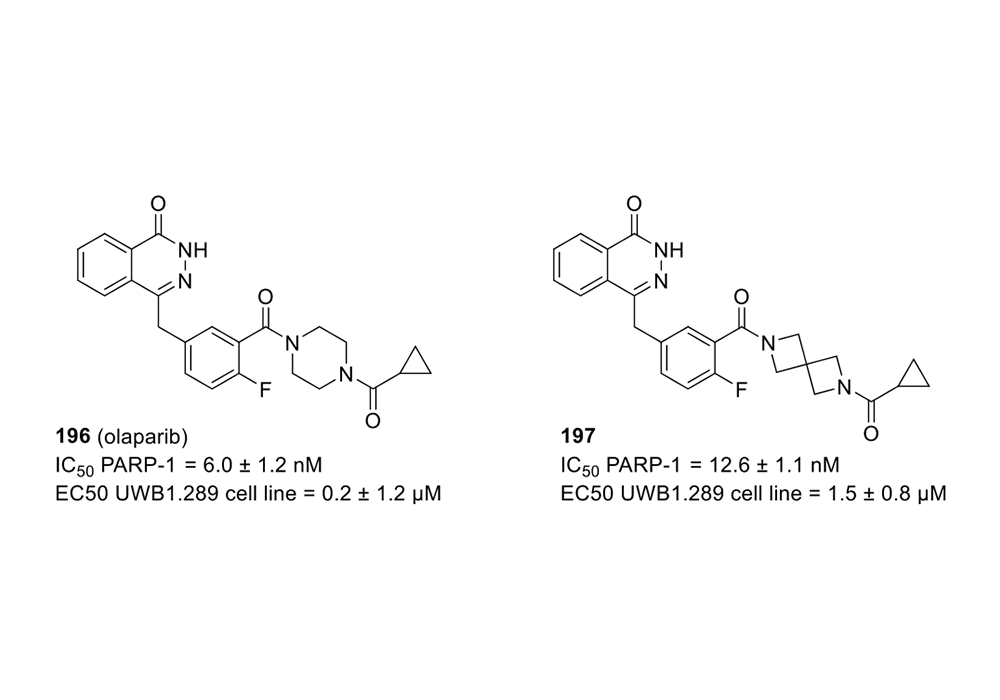
Figure 5. Replacement of piperazine by diazaspiro [3.3] heptane in Olaparib
Therefore, the introduction of spirocyclic scaffolds leads to higher Fsp3, improves the compound potency, selectivity, and pharmacokinetic (PK) properties(Hiesinger et al., 2021).
References
[1]Bagdanoff, J.T., Chen, Z., Acker, M., Chen, Y.N., Chan, H., Dore, M., Firestone, B., Fodor, M., Fortanet, J., Hentemann, M., et al. (2019). Optimization of Fused Bicyclic Allosteric SHP2 Inhibitors. J Med Chem 62, 1781-1792.
[2]Hiesinger, K., Dar'in, D., Proschak, E., and Krasavin, M. (2021). Spirocyclic Scaffolds in Medicinal Chemistry. J Med Chem 64, 150-183.
[3]Johansson, A., Lofberg, C., Antonsson, M., von Unge, S., Hayes, M.A., Judkins, R., Ploj, K., Benthem, L., Linden, D., Brodin, P., et al. (2016). Discovery of (3-(4-(2-Oxa-6-azaspiro[3.3]heptan-6-ylmethyl)phenoxy)azetidin-1-yl)(5-(4-methoxy phenyl)-1,3,4-oxadiazol-2-yl)methanone (AZD1979), a Melanin Concentrating Hormone Receptor 1 (MCHr1) Antagonist with Favorable Physicochemical Properties. J Med Chem 59, 2497-2511.
[4]Lovering, F., Bikker, J., and Humblet, C. (2009). Escape from flatland: increasing saturation as an approach to improving clinical success. J Med Chem 52, 6752-6756.
[5]Reilly, S.W., Puentes, L.N., Wilson, K., Hsieh, C.J., Weng, C.C., Makvandi, M., and Mach, R.H. (2018). Examination of Diazaspiro Cores as Piperazine Bioisosteres in the Olaparib Framework Shows Reduced DNA Damage and Cytotoxicity. J Med Chem 61, 5367-5379.
[6]Sarver, P., Acker, M., Bagdanoff, J.T., Chen, Z., Chen, Y.N., Chan, H., Firestone, B., Fodor, M., Fortanet, J., Hao, H., et al. (2019). 6-Amino-3-methylpyrimidinones as Potent, Selective, and Orally Efficacious SHP2 Inhibitors. J Med Chem 62, 1793-1802.
[7]Zheng, Y.J., and Tice, C.M. (2016). The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin Drug Discov 11, 831-834.