BLD Insights
Application of Bicyclo[1.1.1]pentane in Drug Development Research
15 December 2021
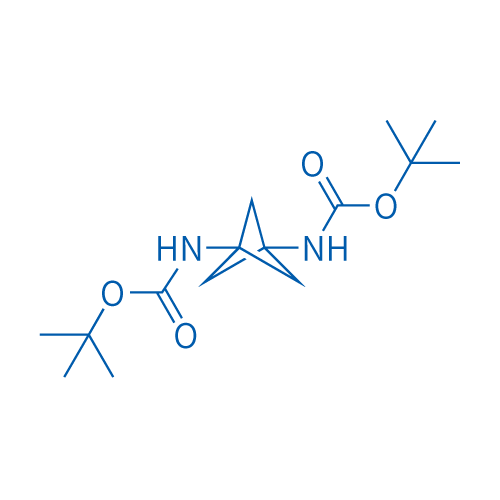
Di-tert-butyl bicyclo[1.1.1]pentane-1,3-diyldicarbamate
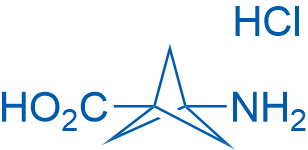
3-Aminobicyclo[1.1.1]pentane-1-carboxylic acid hydrochloride
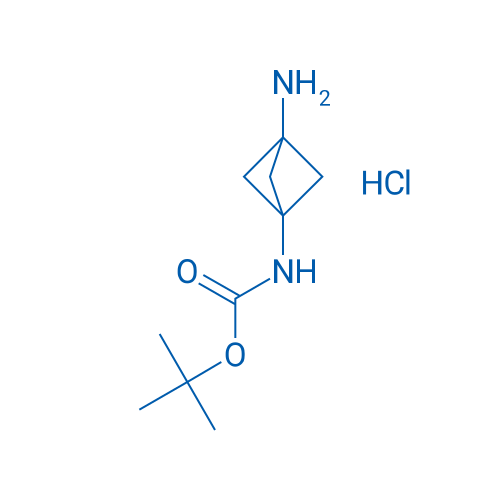
tert-Butyl (3-aminobicyclo[1.1.1]pentan-1-yl)carbamate hydrochloride

Bicyclo[1.1.1]pentane-1,3-dicarboxamide
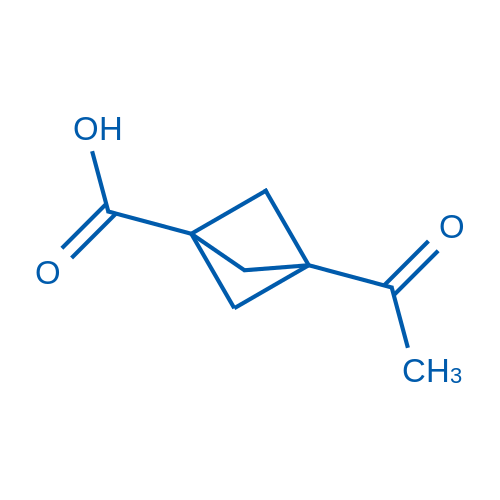
3-Acetylbicyclo[1.1.1]pentane-1-carboxylic acid
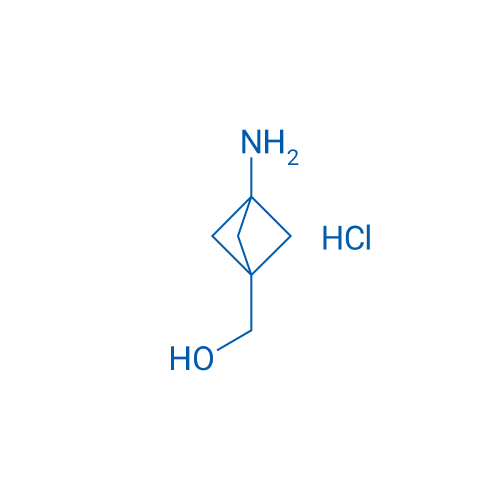
(3-Aminobicyclo[1.1.1]pentan-1-yl)methanol hydrochloride
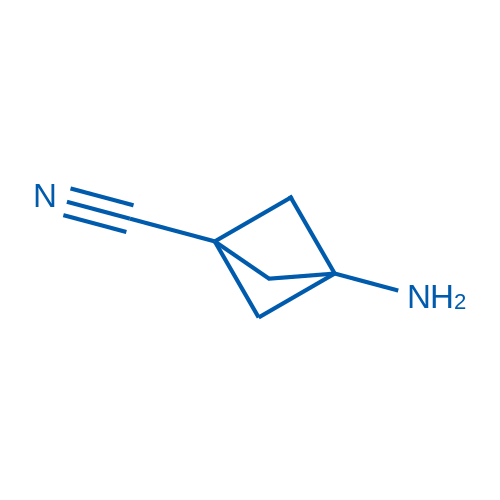
3-Aminobicyclo[1.1.1]pentane-1-carbonitrile
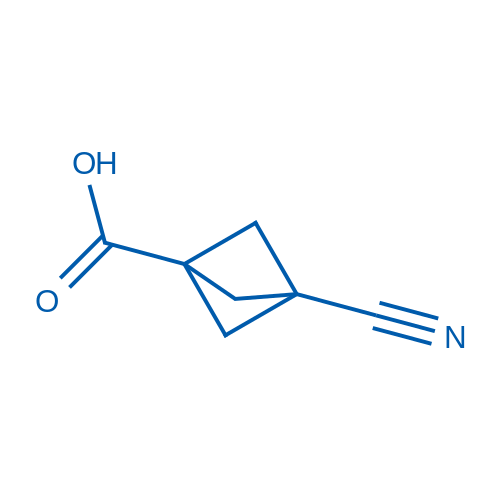
3-cyanobicyclo[1.1.1]pentane-1-carboxylic acid

1,3-Dibromobicyclo[1.1.1]pentane

Di-tert-butyl bicyclo[1.1.1]pentane-1,3-diyldicarbamate

3-Aminobicyclo[1.1.1]pentane-1-carboxylic acid hydrochloride

tert-Butyl (3-aminobicyclo[1.1.1]pentan-1-yl)carbamate hydrochloride

Bicyclo[1.1.1]pentane-1,3-dicarboxamide
Bicyclic [1.1.1] pentane (BCP), containing symmetrical and rigid three-dimensional structure, is used to be a non-classical bioisostere of the phenyl ring in drug research and development. Today, it attracted more attention because of its better pharmaceutical properties, novel structure and rapid development of synthetic methods.1, 2
Figure 1. the common bioisosteric substitution of phenyl ring3, 4
Advantages of BCP
The development of modern chemistry disciplines has perfected the derivatization method based on the phenyl ring. Secondly, the theoretical constructions of the linear free energy theory, the Topliss tree paradigm, the quantitative structure-activity relationship (QSAR) analysis and other drug design concepts are promoting the application of benzene ring in drug development. So that drugs containing phenyl rings account for about 45% of the approved drugs today. However, with the accumulation of practical experience in drug research and development, researchers have also found the benzene ring limitation in drug development because of its poorly physical and chemical properties.
During the last decade, BCP was widely applied in pharmaceutical research and development because of its better physical and chemical properties than phenyl ring: (1) better solubility (2) better metabolic stability (3) more stereo orientation (4) less non-specific binding.1,5-11
A1. More stereo orientation
BCP has similar ring diameter and substituent positions with phenyl ring. So it could simulate the interaction mode of the benzene ring with other amino acid residues, such as π-π stacking. At the same time, the three-dimensional nature of the sp3 carbon atom on the BCP ring makes it possible to explore the pharmacophore or skeleton region orthogonal to the ring plane and has more possibilities of derivatization.
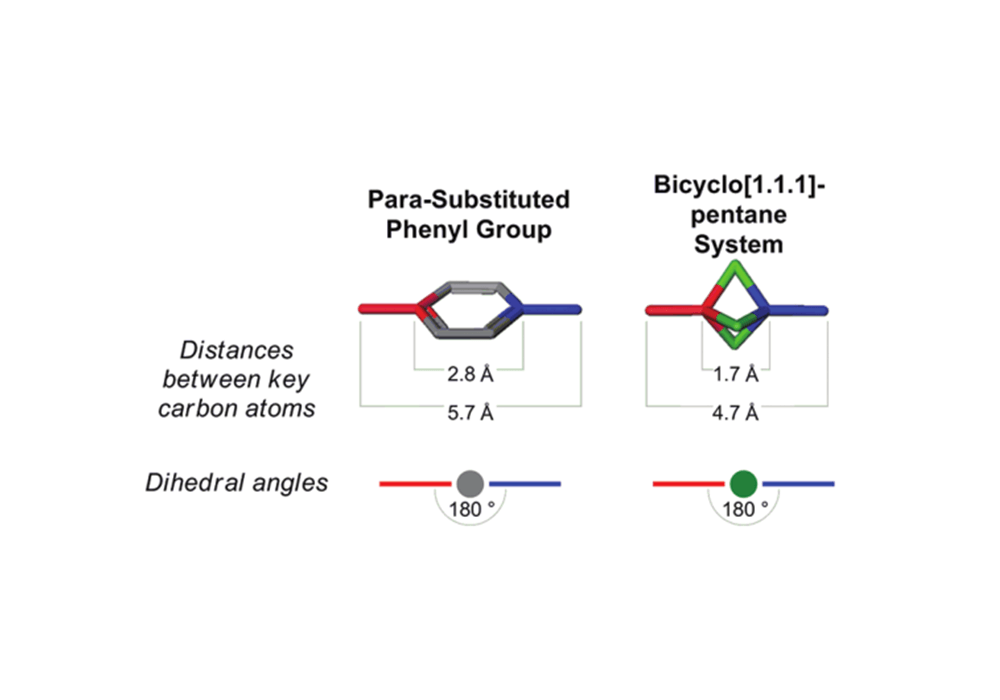
Figure 2. phenyl ring and BCP molecular model
A2. Better solubility and less non-specific binding
The strong π-π interaction formed by the rigid structure and aromaticity of the benzene ring leads to the strong hydrophobicity of the benzene ring, which makes the benzene ring exhibit low solubility in the water phase and has non-specific binding in the hydrophobic environment. In the process of drug screening, the specific binding of drug molecules to target proteins is an important step for drug molecules to interact with target proteins and regulate their protein functions and exert their physiological effects. Due to hydrophobic interactions, van der Waals forces and other factors, the non-specific binding among molecules and biological macromolecules is an important factor for false positive results and off-target effects.12 All of these have affected the pharmacokinetics and pharmacodynamic properties of candidate drugs. Related research is still committed to solving and using non-specific interactions in the in vivo system to promote drug screening and efficient identification of biosensors. Emmanuelle Briard's research team conducted related studies and found that the use of BCP groups to replace the benzene ring can effectively improve the solubility and non-specific binding of the molecule. As shown in Figure 4, after the compound of Figure 3 is replaced with a BCP ring from a benzene ring, the non-specific binding tendency (CHI value) is greatly reduced.1
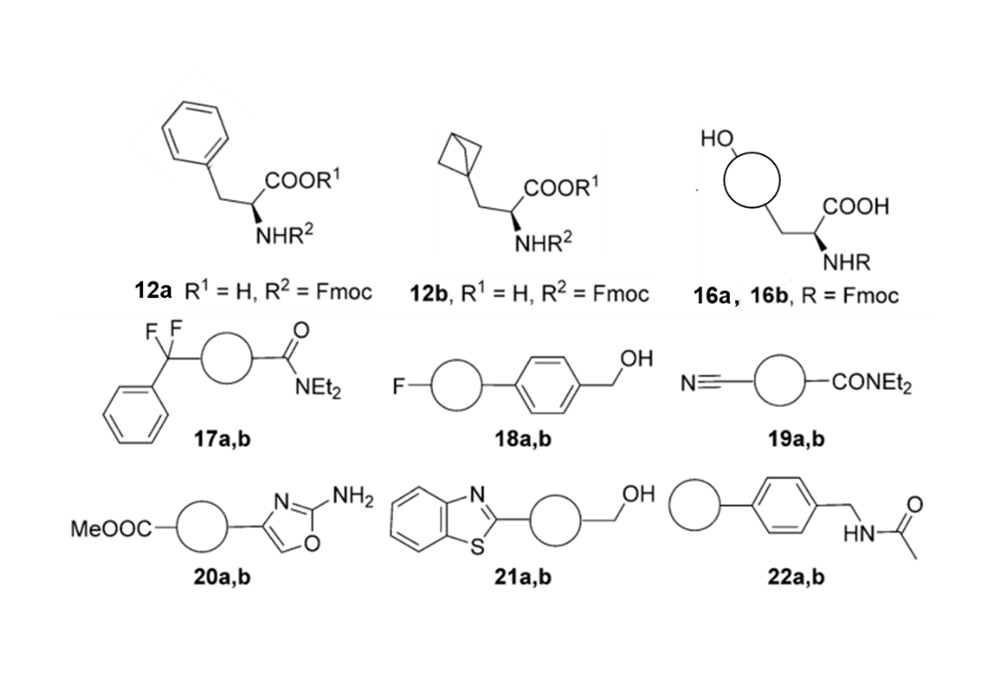
Figure 3. Phenyl ring (a series) and BCP substituted analogs (b series)
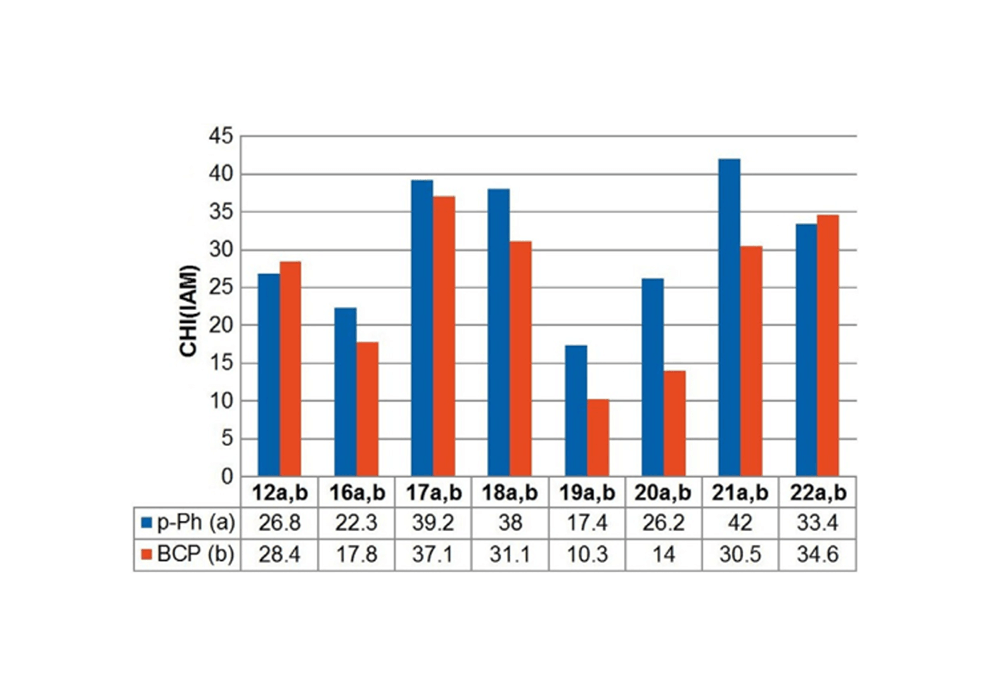
Figure 4. Comparison of CHI (IAM) values between benzene ring (a series) and BCP
substituted analogs (b series): The chromatographic hydrophobicity index CHI (IAM) determined by experiments on immobilized artificial membranes is related to molecular non-specific binding. It is a standardized value derived from the retention time in a high performance liquid chromatography (HPLC) column and was originally developed to characterize the interaction of drugs with immobilized artificial membranes. Therefore, CHI(IAM) can be used to quantify the tendency of chemical substances to bind to molecules non-specifically and allow direct comparison of molecular pairs. The lower the CHI (IAM) value, the weaker the tendency for non-specific binding.
A3. Better metabolic stability
The saturated carbon atoms of the BCP ring are more stable in the CYP450 environment, and the metabolic intermediates and products are less cytotoxic. The metabolic pathways and intermediates of the benzene ring are shown in Figure 5, and the mechanism of BCP metabolism is shown in Figure 6.
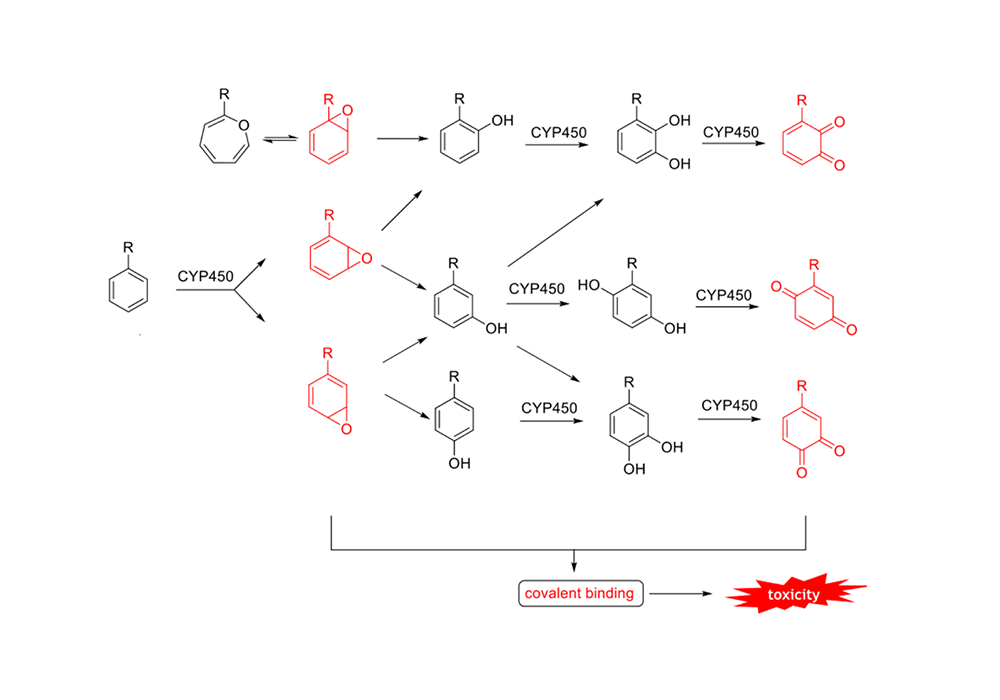
Figure 5. Intermediates of CYP450-mediated benzene ring metabolism
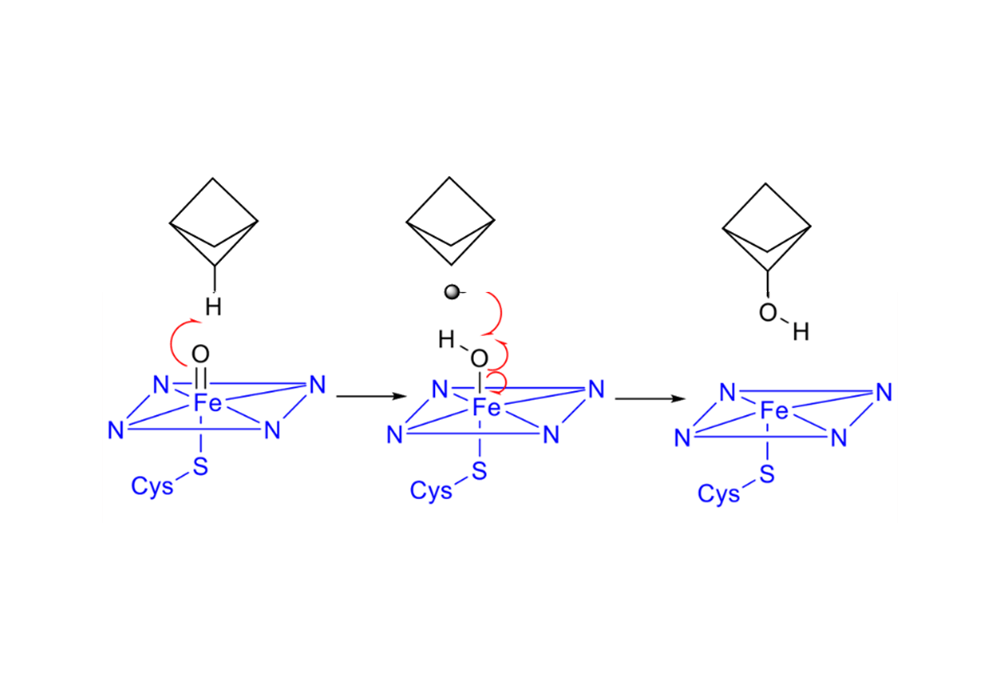
Figure 6. The free radical reaction mechanism of the hydroxylation of BCP by CYP450 enzymes
Application case of BCP in drug design and development
BCP-based building blocks can be effectively used to construct different target molecules, which greatly facilitates the development and industrial application of corresponding drug/natural product analogs. Depended on BCP modification, new drugs with better in vivo and in vitro activities and drug-like properties can be developed more quickly.
Among them, the earliest reported successful case of BCP in drug design was that Pfizer replaced the benzene ring of the γ-secretase inhibitor BMS-708,163 with BCP, which significantly improved the solubility, cell membrane permeability and metabolic stability while maintaining the cell activity of the compound in 2012. As shown in Figure 7. In recent years, BCP application cases reported in related patents and literature have also increased sharply, as shown in Figure 8.
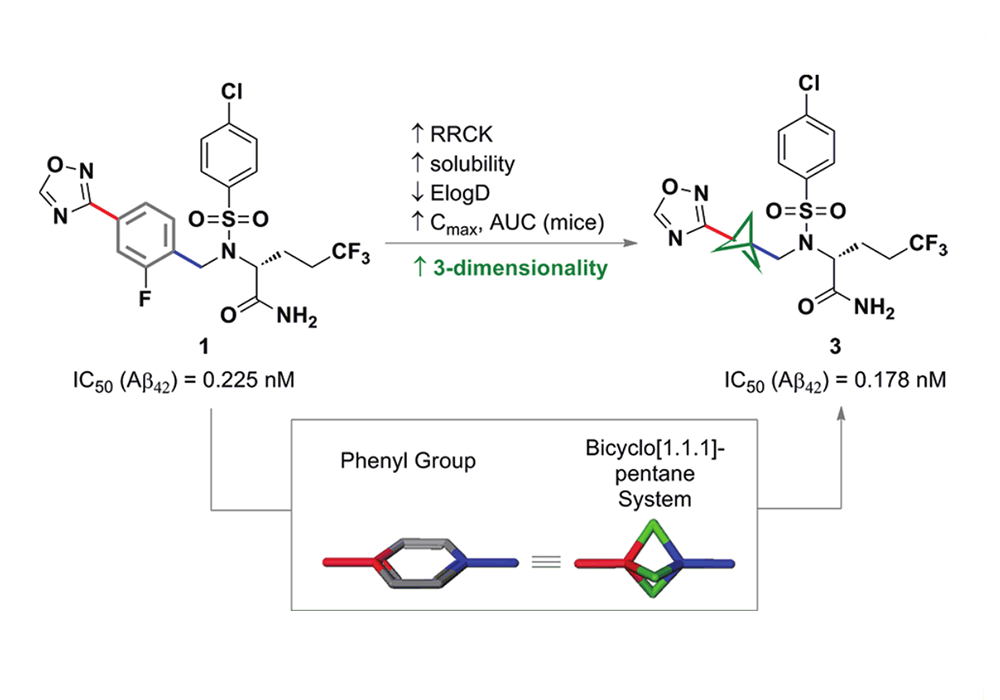
Figure 7. 2012, Pfizer released a potent orally active γ-secretase inhibitor
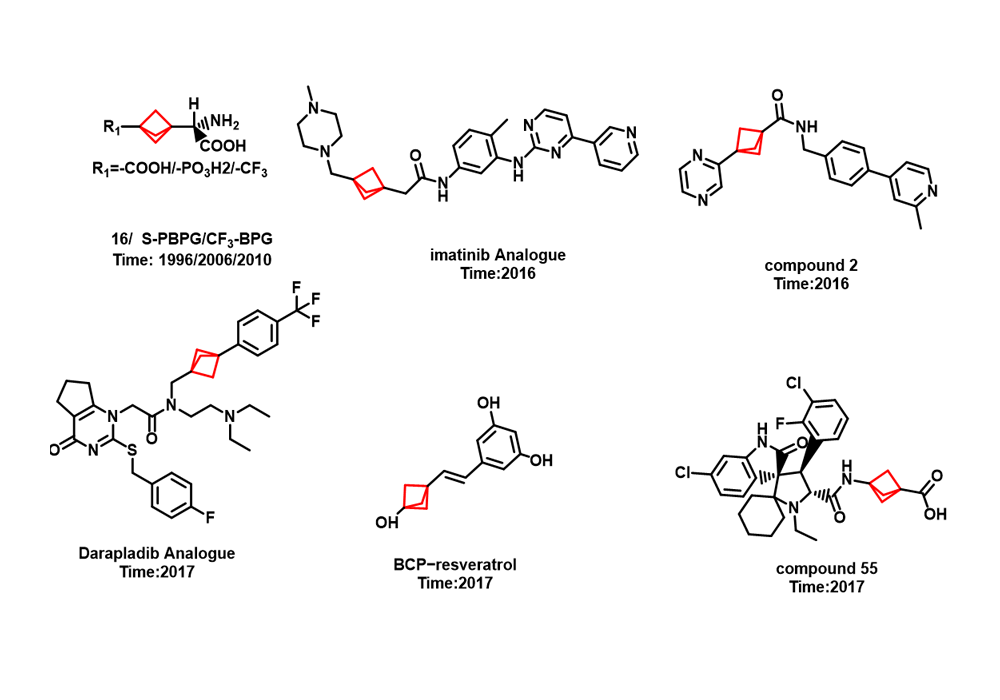
Figure 8. Other examples6-8
References
[1]Auberson, Y. P.; Brocklehurst, C.; Furegati, M.; Fessard, T. C.; Koch, G.; Decker, A.; La Vecchia, L.; Briard, E., Improving Nonspecific Binding and Solubility: Bicycloalkyl Groups and Cubanes as para-Phenyl Bioisosteres. ChemMedChem 2017, 12, 590-598.
[2]Mykhailiuk, P. K., Saturated bioisosteres of benzene: where to go next? . Organic & Biomolecular Chemistry 2019; Vol. 17.
[3]Subbaiah, M. A. M.; Meanwell, N. A., Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design. Journal of medicinal chemistry 2021, 64, 14046-14128.
[4]Tse, E. G.; Houston, S. D.; Williams, C. M.; Savage, G. P.; Rendina, L. M.; Hallyburton, I.; Anderson, M.; Sharma, R.; Walker, G. S.; Obach, R. S.; Todd, M. H., Nonclassical Phenyl Bioisosteres as Effective Replacements in a Series of Novel Open-Source Antimalarials. Journal of medicinal chemistry 2020, 63, 11585-11601.
[5]Stepan, A. F.; Subramanyam, C.; Efremov, I. V.; Dutra, J. K.; O'Sullivan, T. J.; DiRico, K. J.; McDonald, W. S.; Won, A.; Dorff, P. H.; Nolan, C. E.; Becker, S. L.; Pustilnik, L. R.; Riddell, D. R.; Kauffman, G. W.; Kormos, B. L.; Zhang, L.; Lu, Y.; Capetta, S. H.; Green, M. E.; Karki, K.; Sibley, E.; Atchison, K. P.; Hallgren, A. J.; Oborski, C. E.; Robshaw, A. E.; Sneed, B.; O'Donnell, C. J., Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active gamma-secretase inhibitor. Journal of medicinal chemistry 2012, 55, 3414-24.
[6]Pellicciari, R.; Raimondo, M.; Marinozzi, M.; Natalini, B.; Costantino, G.; Thomsen, C., (S)-(+)-2-(3'-Carboxybicyclo[1.1.1]pentyl)- glycine, a Structurally New Group I Metabotropic Glutamate Receptor Antagonist. Journal of medicinal chemistry 1996, 39, 2874-2876.
[7]Filosa, R.; Marinozzi, M.; Costantino, G.; Hermit, M. B.; Thomsen, C.; Pellicciari, R., Synthesis and biological evaluation of (2S)- and (2R)-2-(3′-phosphonobicyclo[1.1.1]pentyl)glycines as novel group III selective metabotropic glutamate receptor ligands. Bioorganic & Medicinal Chemistry 2006, 14, 3811-3817.
[8]Mykhailiuk, P. K.; Voievoda, N. M.; Afonin, S.; Ulrich, A. S.; Komarov, I. V., An optimized protocol for the multigram synthesis of 3-(trifluoromethyl)bicyclo[1.1.1]pent-1-ylglycine (CF3-Bpg). Journal of Fluorine Chemistry 2010, 131, 217-220.
[9]Measom, N. D.; Down, K. D.; Hirst, D. J.; Jamieson, C.; Manas, E. S.; Patel, V. K.; Somers, D. O., Investigation of a Bicyclo[1.1.1]pentane as a Phenyl Replacement within an LpPLA2 Inhibitor. ACS medicinal chemistry letters 2017, 8, 43-48.
[10]Goh, Y. L.; Cui, Y. T.; Pendharkar, V.; Adsool, V. A., Toward Resolving the Resveratrol Conundrum: Synthesis and in Vivo Pharmacokinetic Evaluation of BCP–Resveratrol. ACS medicinal chemistry letters 2017, 8, 516-520.
[11]Aguilar, A.; Lu, J.; Liu, L.; Du, D.; Bernard, D.; McEachern, D.; Przybranowski, S.; Li, X.; Luo, R.; Wen, B.; Sun, D.; Wang, H.; Wen, J.; Wang, G.; Zhai, Y.; Guo, M.; Yang, D.; Wang, S., Discovery of 4-((3′R,4′S,5′R)-6″-Chloro-4′-(3-chloro-2-fluorophenyl)-1′-ethyl-2″-oxodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indoline]-5′-carboxamido)bicyclo[2.2.2]octane-1-carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development. Journal of medicinal chemistry 2017, 60, 2819-2839.
[12]Frutiger, A.; Tanno, A.; Hwu, S.; Tiefenauer, R. F.; Vörös, J.; Nakatsuka, N., Nonspecific Binding—Fundamental Concepts and Consequences for Biosensing Applications. Chemical Reviews 2021, 121, 8095-8160.