BLD Insights
Glycosidation - Critical Process of Carbohydrate Chemistry
06 January 2022
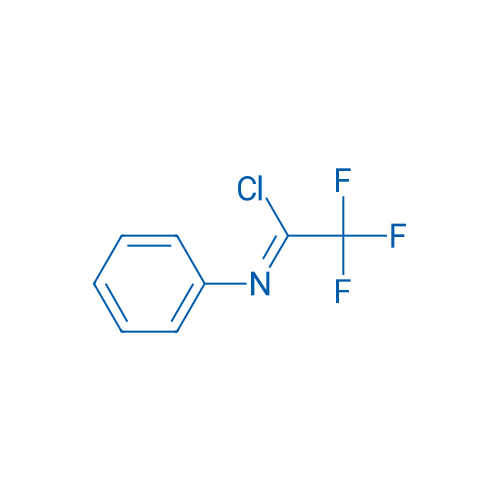
2,2,2-Trifluoro-N-phenylacetimidoyl chloride
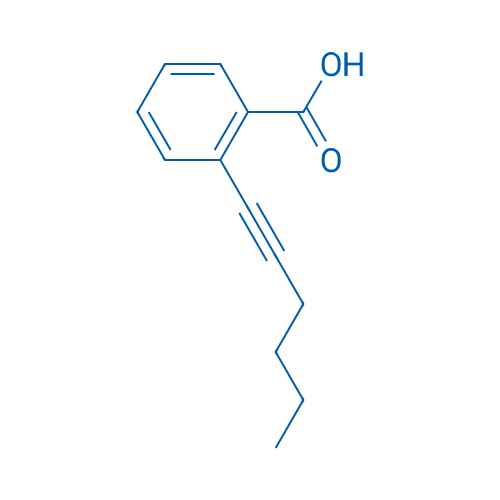
2-(Hex-1-yn-1-yl)benzoic acid

2,3,4,6-Tetra-O-acetyl-b-D-glucopyranosyl trichloroacetimidate
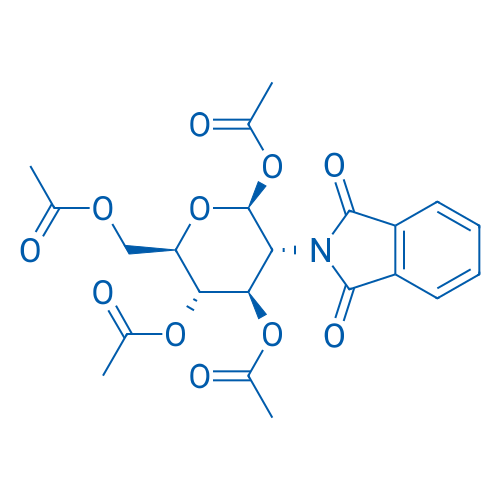
1,3,4,6-Tetra-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranose

2,2,2-Trifluoro-N-phenylacetimidoyl chloride

2-(Hex-1-yn-1-yl)benzoic acid
Glycosidation is the most critical reaction in the synthesis of sugar compounds and sugar-modified drugs. The highly regioselective and stereoselective construction of glycosidic bonds can be widely used in the synthesis of carbohydrates: (1) realizing the assembly or modification of carbohydrate structural units, which could solve the problem of difficult extraction and separation of carbohydrates in natural products; (2) promoting the research and application of compounds in the fields of biology, medicine, and pharmacy. Today, there are mainly the following methods of glycosidation:
Base / Heavy metal salts catalyze glycosidation
Koenigs–Knorr reaction
The synthesis of glycosides by using glycosyl halides and strong bases was first reported by A. Michael in 1879. Dozens of years after, 1901, W. Koenigs and E. Knorr improved this reaction through heavy metal salts or Lewis acids. It is widely used in the synthesis of glycosides with complex groups attached to anomeric carbon atoms, especially oligosaccharides (Fig.1). 1 2The reaction ratio of α/β is determined by various factors such as steric hindrance of the substituents, catalysts, ligands, ortho Substituents, and basic properties.
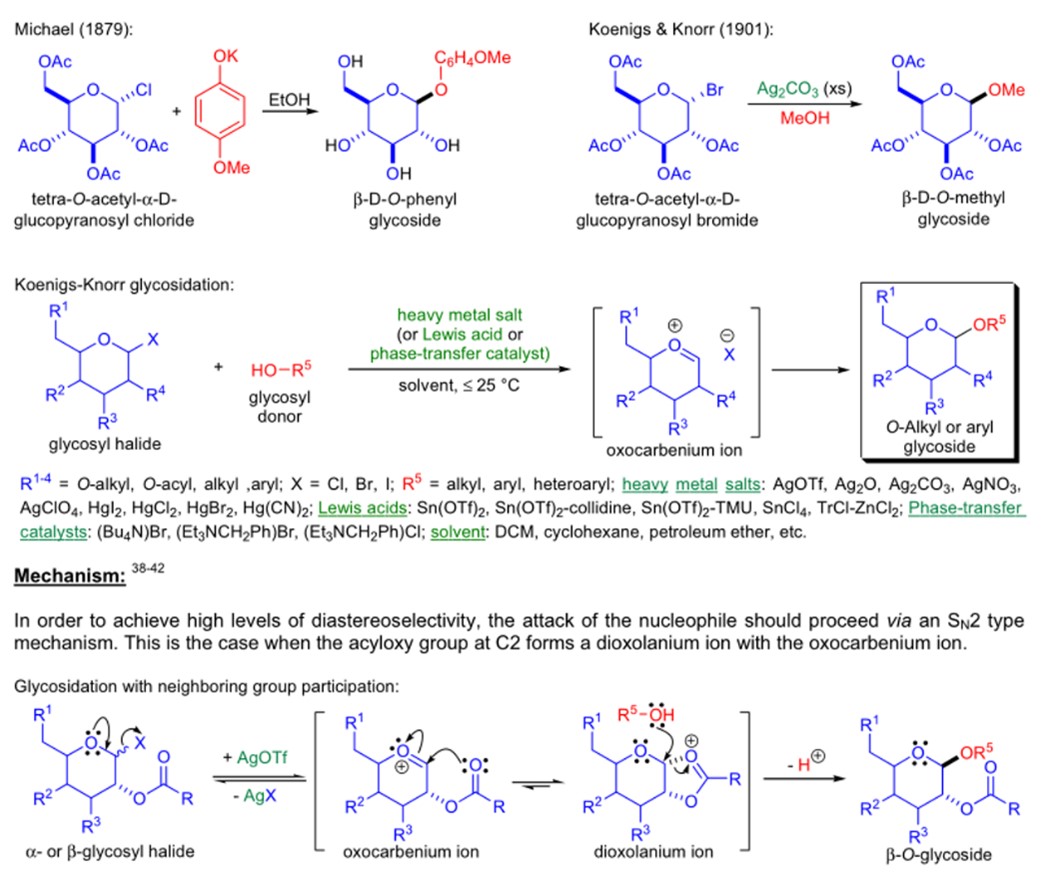
Figure 1: Koenigs–Knorr reaction&mecanism
Kahne–Crich glycosidation
Synthesis of glycosides, disaccharides, or oligosaccharides by reaction between glycosyl phenyl sulfoxide and an acceptor in the presence of a glycosylation promoter (Fig.2). 3
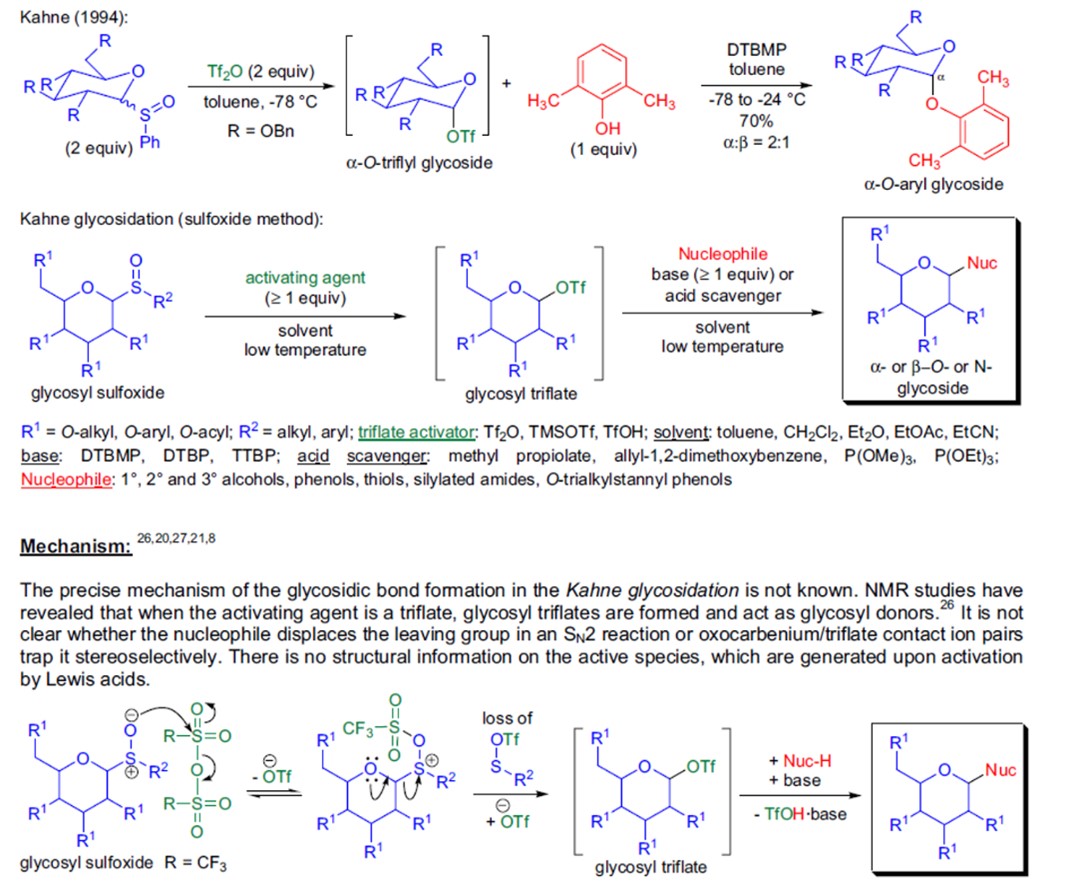
Figure 2: Kahne-Crich glycosidation
Protonic acids / Lewis acids catalyze glycosidation
Fischer-Helferich glycosidation
Fischer achieved the formation of a glycoside by the reaction of an aldose or ketose with an alcohol in the presence of an acid catalyst during the late 19th. Helferich and others modified the reaction later by using a glycosyl acetate or glycosyl halide as glycosyl donor, with a Lewis acid/ a mercury salt as a promoter (Fig.3). 4 5
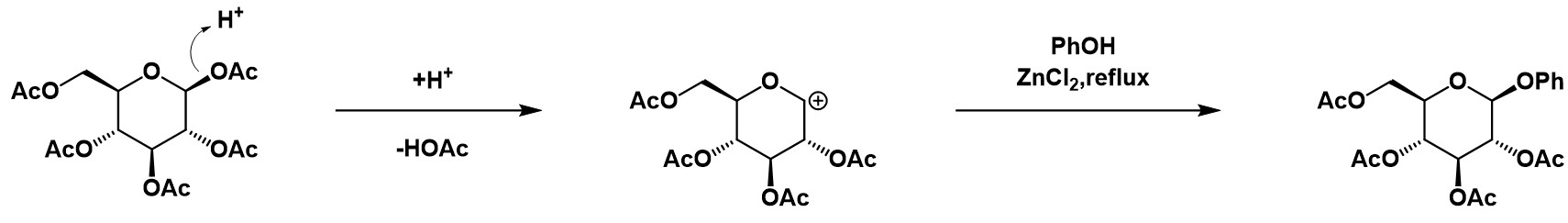
Figure 3: Fischer-Helferich glycosidation
Schmidt Glycosylation
The Schmidt glycosylation is a Lewis acid-catalyzed glycosylation using glycosyl trichloroacetimidate as the donor (Fig.4a). 6 2001, Yu lab modified the reaction by using Glycosyl (N-phenyl) trifluoroacetimidates as effective glycosyl donors (Fig.4b). 7 2021, Peng lab adjusted the lewis acid to achieve stereoselective and regioselective glycosidation (Fig.4c). 8
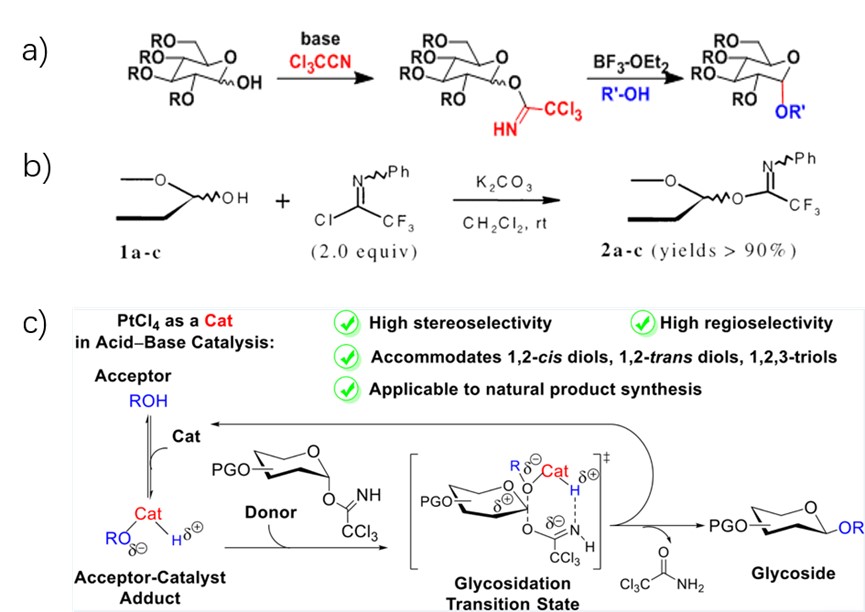
Figure 4: Schmidt Glycosylation (a) & modification (b/c)
Metal coupling reagents catalyze glycosidation
Yu glycosidation (Au-catalyzed)
2008, Yu lab applied the recently developed chemistry of alkynylophilic gold(I) catalysts to the development of new glycosylation reactions that would avoid the use of the conventional leaving groups and promoters (Fig.5a).9 Then, Sun lab established a novel alkyne-activation-based glycosylation protocol using o-(p-methoxyphenylethynyl)phenyl (MPEP) glycoside depending on Yu lab's study (Fig.5b). 10
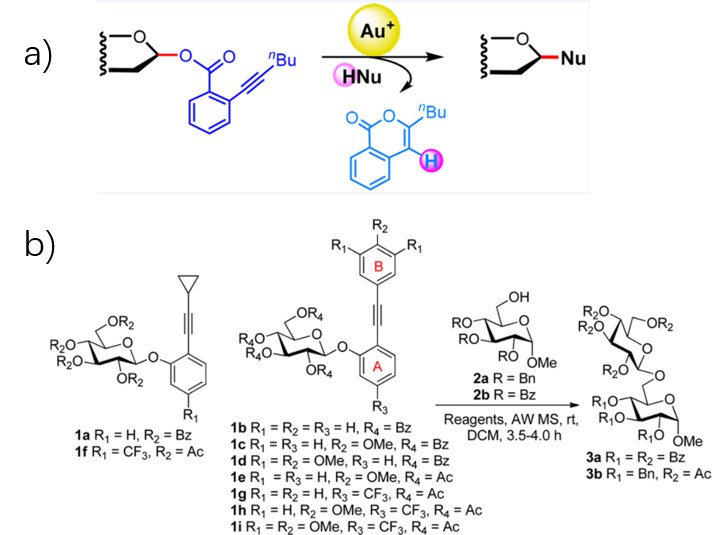
Figure 5: Yu glycosidation(a) & Sun lab’s glycosidation(b)
Glycosidation based on Pd-catalyzed coupling reaction
Palladium-catalyzed coupling is an important method in the field of metal-catalyzed coupling. It can be applied to the construction of various target products. Therefore, it is also widely used in glycosidation (Fig.6).11, 12
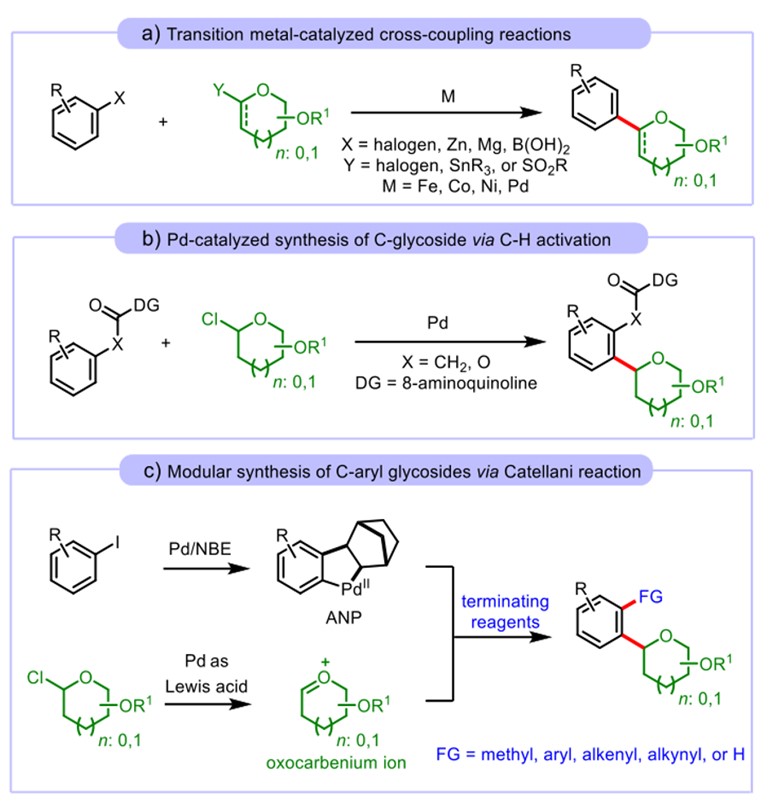
Figure 6: Glycosidation based on (a) Tsuji-Trost reaction, (b) C-H active coupling, and (c) Catellani reaction
References
[1]Holman, R. W. Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms (Kürti, László; Czakó, Barbara). Journal of Chemical Education, 2005, 82, p246.
[2]Igarashi, K., The Koenigs-Knorr Reaction. Advances in Carbohydrate Chemistry and Biochemistry, 1970; Vol. 34.
[3]Holman, R. W. Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms (Kürti, László; Czakó, Barbara). Journal of Chemical Education, 2005, 82, p234
[4]Khadem, H. S. E., Carbohydrates. Encyclopedia of Physical Science and Technology (Third Edition),2003.
[5]Priyanka Bose*, A. K. A., Anoop S. Singh, Manoj K. Jaiswal, Vinod K. Tiwari, Sialic acid-containing molecules in drug discovery and development. Carbohydrates in Drug Discovery and Development, 2020,5.
[6]Schmidt, R. R., New Methods for the Synthesis of Glycosides and Oligosaccharides—Are There Alternatives to the Koenigs-Knorr Method? [New Synthetic Methods (56)]. Angewandte Chemie International Edition in English, 1986, 25, 212-235.
[7]Yu, B.; Tao, H., Glycosyl trifluoroacetimidates. Part 1: Preparation and application as new glycosyl donors. Tetrahedron Letters, 2001, 42, 2405-2407.
[8]Li, T.; Li, T.; Zhuang, H.; Wang, F.; Schmidt, R. R.; Peng, P., O-Glycosyl Trichloroacetimidates as Glycosyl Donors and Platinum(IV) Chloride as a Dual Catalyst Permitting Stereo- and Regioselective Glycosidations. ACS Catalysis , 2021, 11, 10279-10287.
[9]Yu, B., Gold(I)-Catalyzed Glycosylation with Glycosyl o-Alkynylbenzoates as Donors. Accounts of Chemical Research, 2018, 51, 507-516.
[10]Hu, Y.; Yu, K.; Shi, L.-L.; Liu, L.; Sui, J.-J.; Liu, D.-Y.; Xiong, B.; Sun, J.-S., o-(p-Methoxyphenylethynyl)phenyl Glycosides: Versatile New Glycosylation Donors for the Highly Efficient Construction of Glycosidic Linkages. Journal of the American Chemical Society , 2017, 139, 12736-12744.
[11]Dai, Y.; Zheng, J.; Zhang, Q., General Strategy for Stereoselective Synthesis of β-N-Glycosyl Sulfonamides via Palladium-Catalyzed Glycosylation. Organic Letters, 2018, 20, 3923-3927.
[12]Lv, W.; Chen, Y.; Wen, S.; Ba, D.; Cheng, G., Modular and Stereoselective Synthesis of C-Aryl Glycosides via Catellani Reaction. Journal of the American Chemical Society, 2020, 142, 14864-14870.