How BLD Helps
ARV-471 (Vepdegestrant): a PROTAC Estrogen Receptor Degrader for Breast Cancer
17 July 2024
Arvinas and Pfizer are collaborating to develop and commercialize ARV-471, an investigational PROTAC estrogen receptor degrader administered orally for the treatment of patients with estrogen receptor (ER) positive/human epidermal growth factor receptor 2 (HER2) negative (ER+/HER2-) locally advanced or metastatic breast cancer. This program is currently in Phase 3 clinical studies.
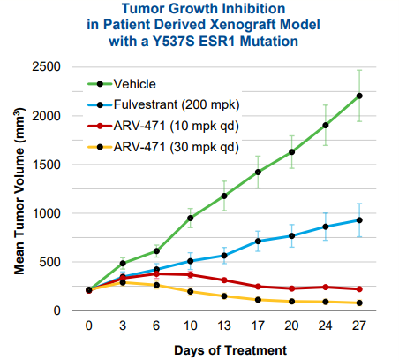
Preclinical studies of ARV-471 have been published[1]. It has better tumor inhibition effect compared with ER antagonist Fulvestrant, and oral, daily dose of ARV-471 inhibited tumor growth by 99% at 10 mpk and 106% at 30 mpk in an ESR1 mutant PDX model.
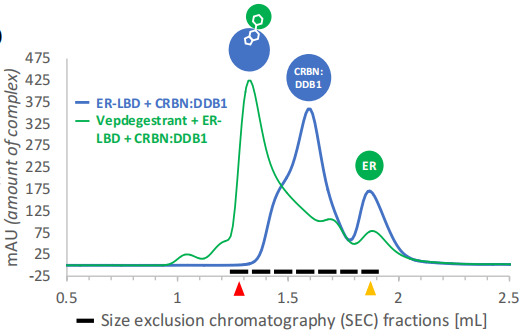
The individual ER and CRBN: DDB1 components were mixed in excess and incubated with Vepdegestrant (green line) and without Vepdegestrant (blue line), then separated using size-exclusion chromatography the size-exclusion. The results were shown in figure [2], which verified the formation of a ternary complex in the degradation mechanism of PROTAC.
Phase 2 clinical data of ARV-471 were published in November 2022[3], the CBR(CR+PR+ SD≥24w) of the primary endpoint for the ESR1 mutation subgroup was as high as 51%, and the PFS of the ESR1 mutation subgroup was 5.7 months. Safety studies showed that ARV-471 was well tolerated at both doses, and the most common (≥10%) TRAEs were nausea (27%), fatigue (20%), and vomiting (10%), all of which were grade 1.
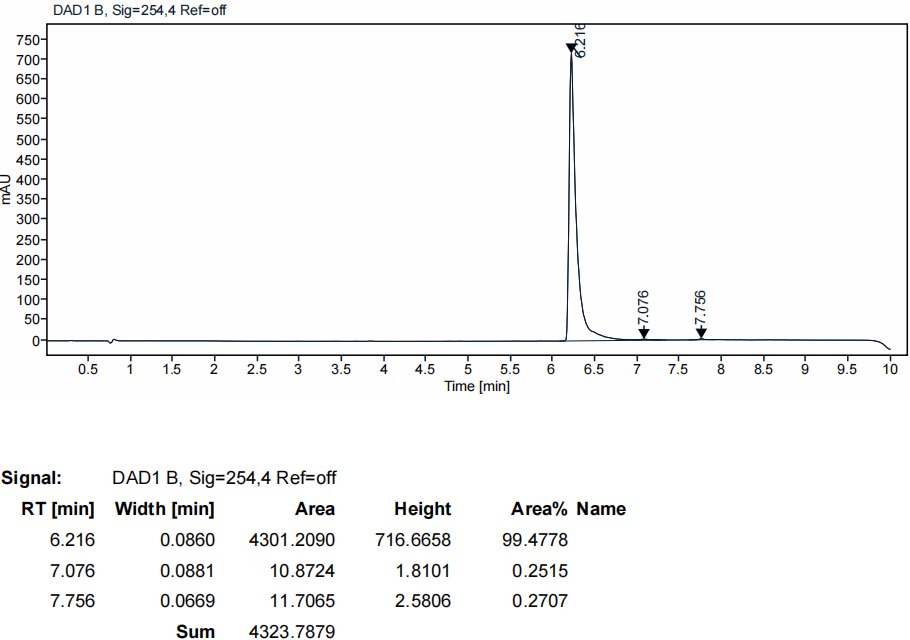
HPLC of our products
References
[1]AACR Annual Meeting,2021: APRIL 10-15, 2021 AND MAY 17-21, 2021.
[2]Clin Cancer Res. 2024 May 31. doi: 10.1158/1078-0432.
[3]San Antonio Breast Cancer Symposium®, December 6–10, 2022, Presentation GS3-03.