How to influence cell fate?
27 June 2024
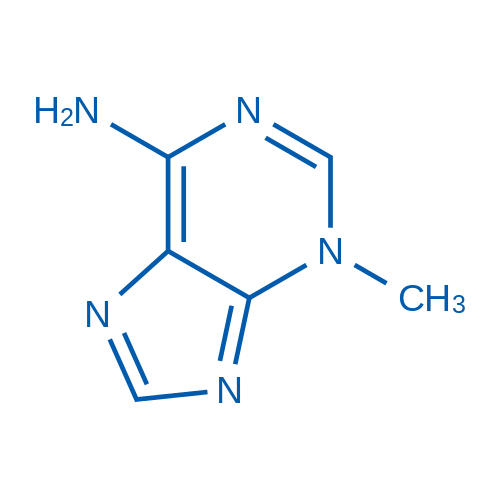
3-Methyl-3H-purin-6-amine
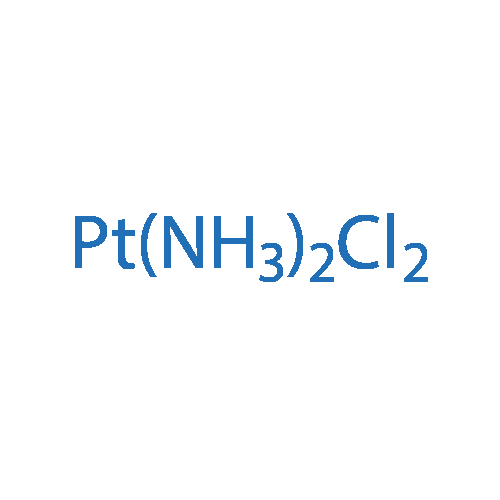
cis-Diaminodichloroplatinum(II)
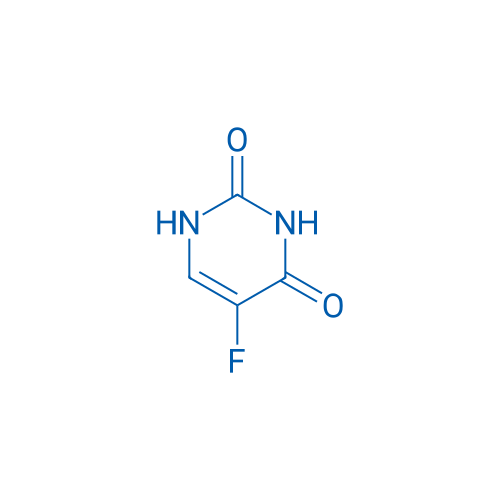
5-Fluoropyrimidine-2,4(1H,3H)-dione
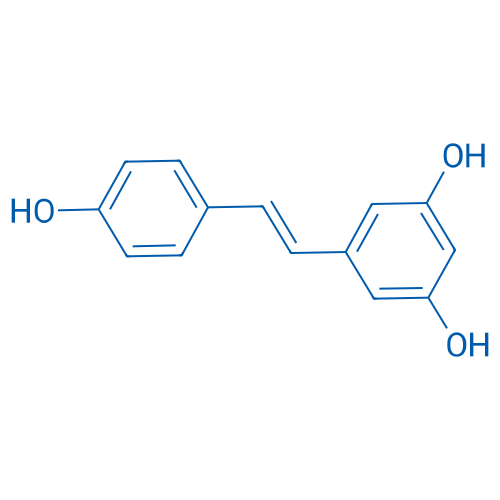
(E)-5-(4-Hydroxystyryl)benzene-1,3-diol
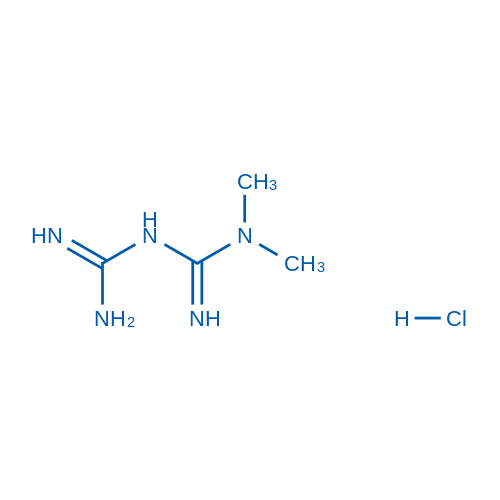
1,1-Dimethylbiguanide Hydrochloride

3-Methyl-3H-purin-6-amine
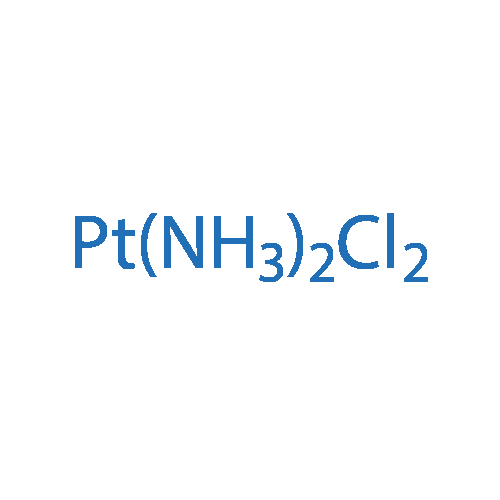
cis-Diaminodichloroplatinum(II)
In the grand ballet of life, cells pirouette on the stage of existence, their survival precariously balanced by a myriad of external and internal cues. Infections, metabolic dysregulation, or the spontaneous choreography of development can lead to a dramatic finale—a variety of cellular death forms, including programmed cell death (apoptosis and autophagy), necroptosis, pyroptosis, ferroptosis, cuproptosis, lysosomal cell death, oxeiptosis, PANoptosis, entosis, NETosis, alkaliptosis, and parthanatos. Among these, apoptosis, autophagy, ferroptosis, pyroptosis, and cuproptosis have been the most extensively studied[1].
To facilitate the study of cell death, the use of tool compounds to trigger or inhibit various forms of cell death is essential. In this issue, BLD provides a compendium of commonly used tool compounds:
Apoptosis:
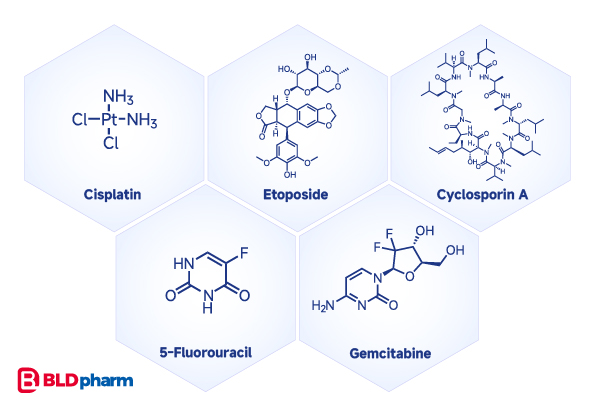
Many compounds can induce apoptosis, targeting key proteins or nodes in the apoptotic process. These include DNA-damaging agents like cisplatin, topoisomerase II inhibitors such as etoposide, calcineurin inhibitors like Cyclosporine A, nucleotide analogs 5-fluorouracil, gemcitabine, and more[2].
Autophagy:
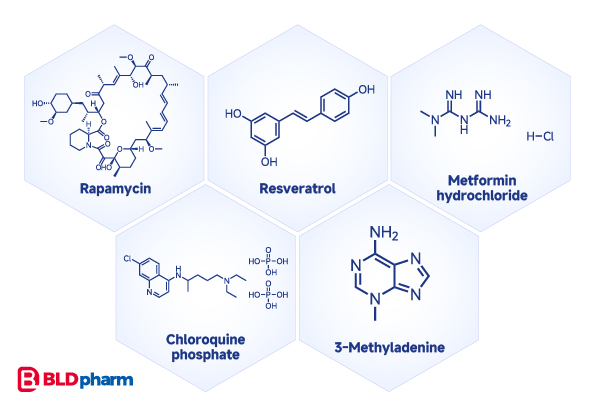
Autophagy, another well-studied cellular physiological process, has its own set of inducers, including Rapamycin and Resveratrol, Metformin, etc.; and inhibitors such as Chloroquine, 3-methyladenine, or bafilomycin A1. These compounds alter lysosomal pH, the LC3B-II: LC3B-I ratio, p-AMPK, and BECN1 protein levels[3].
Ferroptosis:
Ferroptosis, a form of cell death studied almost as extensively as programmed cell death, can be induced by compounds like Erastin, RSL3, L-BSO, and various Statin compounds. Their mechanisms include inducing cysteine deprivation, GPX4 inactivation, and GSH depletion[4].
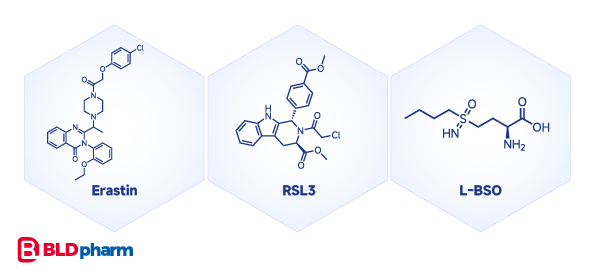
Pyroptosis:
While pyroptosis has been frequently reported, unlike the aforementioned cell death modalities, representative small molecule compounds for pyroptosis induction are scarce, including Nigericin, AC-YVAD-cmk, and disulfiram[5][6].
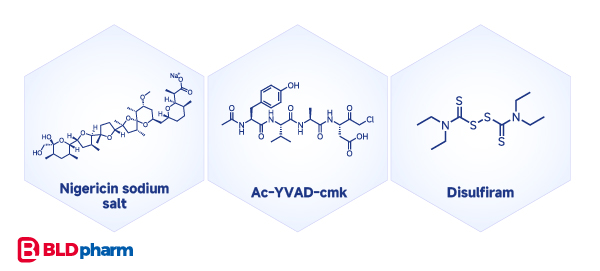
Cuproptosis:
Cuproptosis, a novel form of cell death pathway triggered by copper (Cu), discovered by Peter Tsvetkov and colleagues in 2022, has garnered significant attention, with over 1000 publications since its discovery (data sourced from PubMed). Currently, copper ion chelators such as TTM, trientine, and penicillamineare used to inhibit cuproptosis; while the occurrence of cuproptosis can be induced by copper ion carriers like elesclomol (most frequently used), disulfiram, and bis(thiosemicarbazone) analogs [CuII(atsm) and CuII(gtsm)][7][8][9].
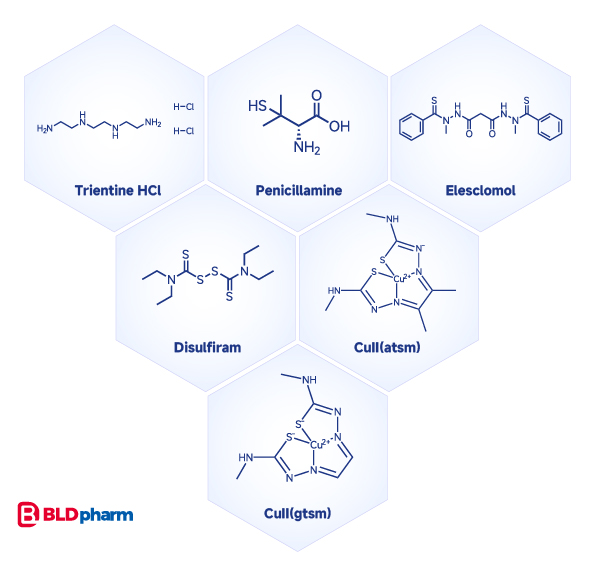
References
[1]Newton K, Strasser A, Kayagaki N, Dixit VM. Cell death. Cell. 2024 Jan 18;187(2):235-256. doi: 10.1016/j.cell.2023.11.044. PMID: 38242081.
[2]Gielecińska A, Kciuk M, Yahya EB, Ainane T, Mujwar S, Kontek R. Apoptosis, necroptosis, and pyroptosis as alternative cell death pathways induced by chemotherapeutic agents? Biochim Biophys Acta Rev Cancer. 2023 Nov;1878(6):189024. doi: 10.1016/j.bbcan.2023.189024. Epub 2023 Nov 18. PMID: 37980943.
[3]Devis-Jauregui L, Eritja N, Davis ML, Matias-Guiu X, Llobet-Navàs D. Autophagy in the physiological endometrium and cancer. Autophagy. 2021 May;17(5):1077-1095. doi: 10.1080/15548627.2020.1752548. Epub 2020 May 13. PMID: 32401642; PMCID: PMC8143243.
[4]Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W, Wang J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med. 2019 Aug;23(8):4900-4912. doi: 10.1111/jcmm.14511. Epub 2019 Jun 24. PMID: 31232522; PMCID: PMC6653007.
[5]Mandell JT, de Rivero Vaccari JP, Sabater AL, Galor A. The inflammasome pathway: A key player in ocular surface and anterior segment diseases. Surv Ophthalmol. 2023 Mar-Apr;68(2):280-289. doi: 10.1016/j.survophthal.2022.06.003. Epub 2022 Jul 4. PMID: 35798189.
[6]Recent progress in pyroptosis probes and inducers
[7]Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, Liu J, Yu X, Shi S. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022 Dec 8;15(1):174. doi: 10.1186/s13045-022-01392-3. PMID: 36482419; PMCID: PMC9733270.
[8]Zou Y, Wu S, Xu X, Tan X, Yang S, Chen T, Zhang J, Li S, Li W, Wang F. Cope with copper: From molecular mechanisms of cuproptosis to copper-related kidney diseases. Int Immunopharmacol. 2024 May 30;133:112075. doi: 10.1016/j.intimp.2024.112075. Epub 2024 Apr 24. PMID: 38663316.
[9]Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022 Nov 23;7(1):378. doi: 10.1038/s41392-022-01229-y. PMID: 36414625; PMCID: PMC9681860.