BLD Insights
Liver-Targeted Delivery System with tri-GalNAc Conjugates
20 June 2022
While small molecules and protein drugs are essential to the success of modern medicine, oligonucleotide (ON) therapeutics have the potential to become a third pillar of drug development. Representative ON-based therapeutics include small interfering RNA (siRNA) that targets and degrades disease-causing mRNA through RNA-induced silencing complex (RISC) mediated RNA interference and antisense oligonucleotide (ASO) that binds complementary mRNA and induce sequence-specific cleavage of the RNA by endonuclease RNase H[1]. However, before siRNAs become drugs, they must overcome evolutionary defenses designed to keep invading RNAs on the outside of cells from getting to the inside of cells, consequently, the problem to solve has remained the same: Delivery!
The development of N-acetylgalactosamine (GalNAc) conjugates, which bind to the Asialoglycoprotein Receptor (ASGPR), has become a breakthrough approach for targeted delivery of therapeutic oligonucleotides to hepatocytes. ASGPR is a high-capacity and rapidly internalizing receptor that is abundantly and specifically expressed on the surface of hepatocytes[2]. GalNAc-nucleic acid is a monoconjugate formed by saccharide compounds and nucleic acid. GalNAc is covalently conjugated to the 3' end of the sense strand of RNA in a trivalent manner to form a polysaccharide-RNA monoconjugates for specific delivery to hepatocytes and drug entry and function via endocytosis[3].
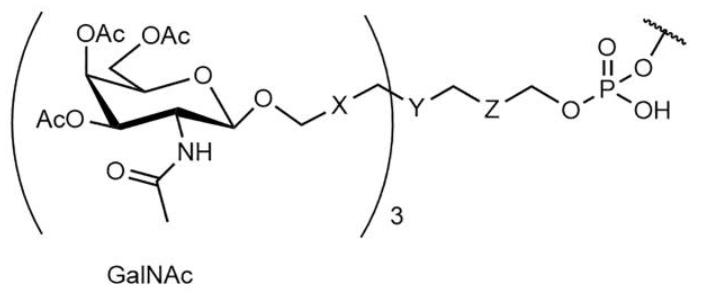
Figure 1. Schemes of the Most Common GalNAc Conjugates[4]
X and Z are commonly ethylene glycol or alkyl spacers. Y is a multifunctional moiety allowing branching of the cluster.
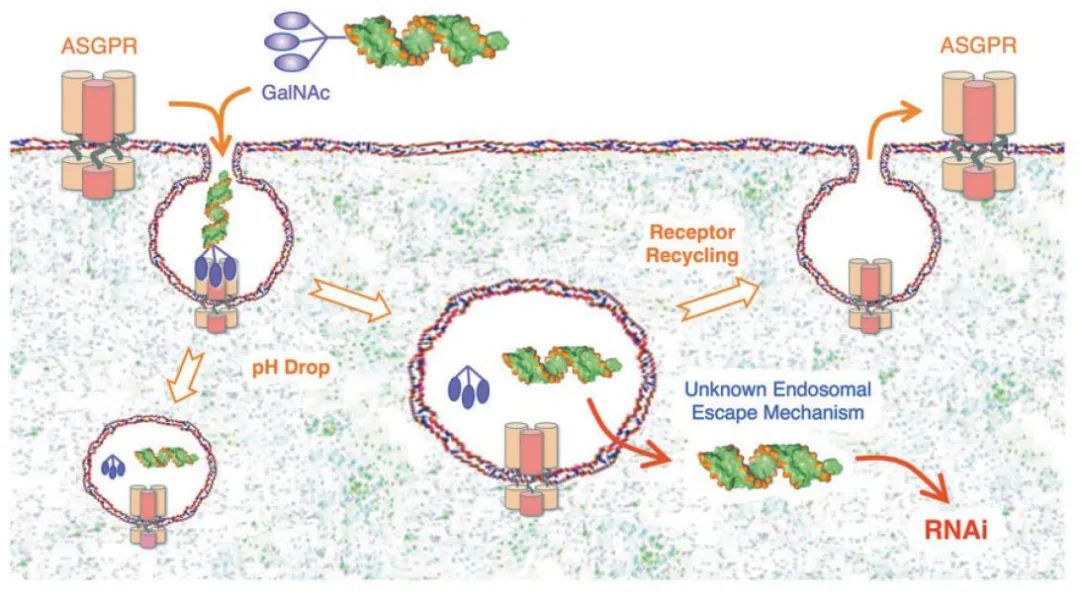
Figure 2. GalNAc-mediated siRNA delivery to liver hepatocytes[4]
To date, GalNAc-based therapeutics feature prominently in the drug development pipelines of several pharma companies, and three GalNAc-siRNA drugs Givosiran, Lumasiran and Inclisiran developed by Alnylam, have achieved their global approval and advanced to clinical applications over the past three years. GalNAc-based drugs in clincal trial developed by the leading pharma companies such as Alnylam, Arrowhead, Dicerna, and Ionis are shown in Table 1.
Table 1. Summary of the Clinical Status of GalNAc Conjugates with Either siRNA or ASO from the Leading Pharma Companies[1]

In the latest study, based on LYsosome TArgeting Chimera (LYTAC), a promising technology to deliver extracellular protein targets to lysosome for degradation through the cation-independent mannose-6-phosphate receptor (CI-M6PR), tri-GalNAc was conjugated to antibodies or fragments of antibodies to generate a new class of degraders. Antibody-based tri-GalNAc degraders noncovalently capture the protein targets and transport the targets to lysosome for degradation via the interaction with ASGPR[5]. This study demonstrated the feasibility of ASGPR-mediated liver cell-specific targeted protein degradation strategy and uncovered a potential new therapeutic application of tri-GalNAc in addition to its well-known utilities in liver-specific delivery of oligonucleotides.
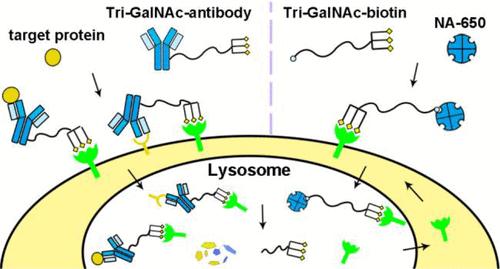
Figure 3. Application of tri-GalNAc in targeted protein degradation[5]
Taken together, tri-GalNAc-based delivery systems open a world of possibilities for the development of potent and liver-targeted oligonucleotide and protein degradation therapies for both rare diseases and common diseases from genetic disorders. As a leading global supplier of small molecule compounds, BLDpharm offers a variety of tri-GalNAc with multiple linkers and reactive handles, which must be a useful tool for tri-GalNAc-based delivery systems research.
References
[1]Cui H, Zhu X, Li S, Wang P, Fang J. Liver-Targeted Delivery of Oligonucleotides with N-Acetylgalactosamine Conjugation. ACS Omega. 2021 Jun 14;6(25):16259-16265. doi: 10.1021/acsomega.1c01755.
[2]D'Souza AA, Devarajan PV. Asialoglycoprotein receptor mediated hepatocyte targeting - strategies and applications. J Control Release. 2015 Apr 10;203:126-39. doi: 10.1016/j.jconrel.2015.02.022.
[3]Debacker AJ, Voutila J, Catley M, Blakey D,Habib N. Delivery of Oligonucleotides to the Liver with GalNAc: From Researchto Registered Therapeutic Drug. Mol Ther. 2020 Aug 5;28(8):1759-1771.
[4]Springer AD, Dowdy SF. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018Jun;28(3):109-118.
[5]Zhou Y, Teng P, Montgomery NT, Li X, Tang W. Development of Triantennary N-Acetylgalactosamine Conjugates as Degraders for Extracellular Proteins. ACS Cent Sci. 2021 Mar 24;7(3):499-506. doi: 10.1021/acscentsci.1c00146.