How BLD Helps
Applications of Oxazoline Ligands in Asymmetric Catalysis
29 June 2022
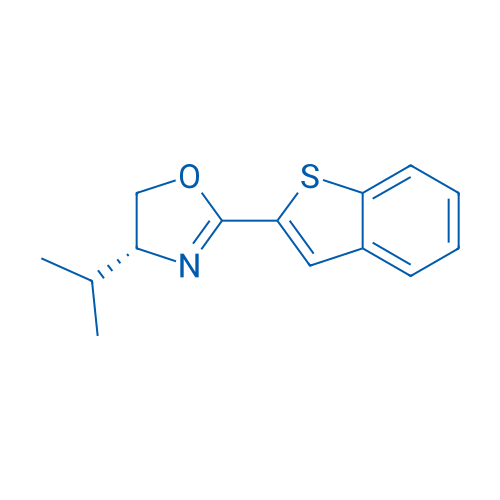
(R)-2-(Benzo[b]thiophen-2-yl)-4-isopropyl-4,5-dihydrooxazole
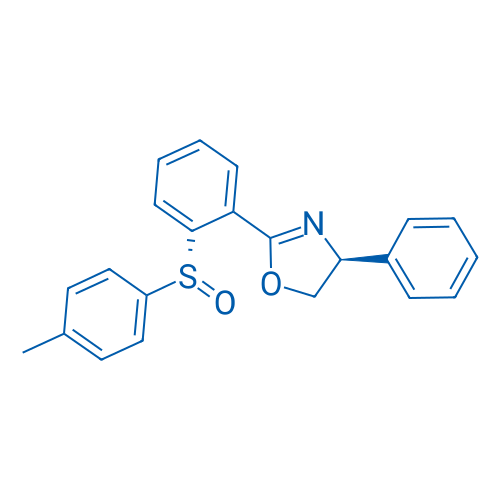
(S)-4-Phenyl-2-(2-((R)-p-tolylsulfinyl)phenyl)-4,5-dihydrooxazole
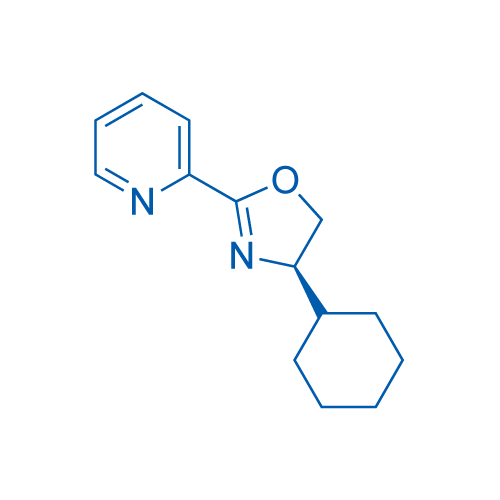
(R)-4-Cyclohexyl-2-(pyridin-2-yl)-4,5-dihydrooxazole
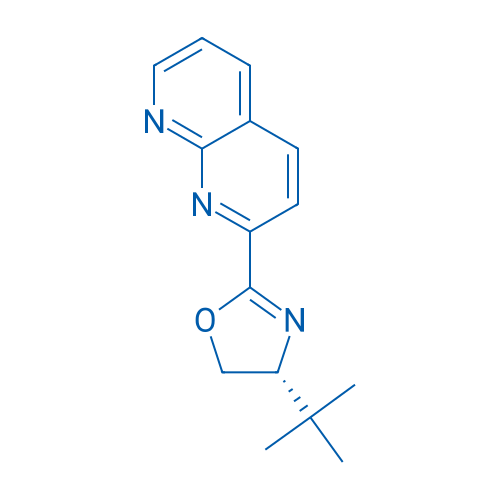
(R)-4-(tert-Butyl)-2-(1,8-naphthyridin-2-yl)-4,5-dihydrooxazole
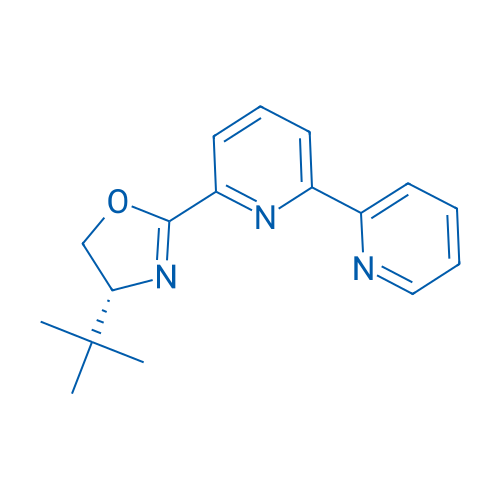
(R)-2-([2,2'-Bipyridin]-6-yl)-4-(tert-butyl)-4,5-dihydrooxazole
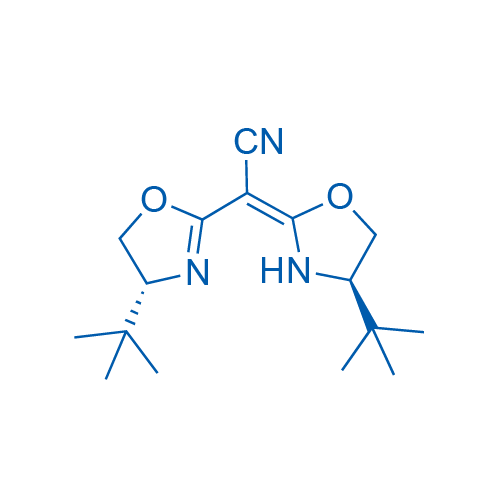
2,2-Bis((R)-4-(tert-butyl)-4,5-dihydrooxazol-2-yl)acetonitrile
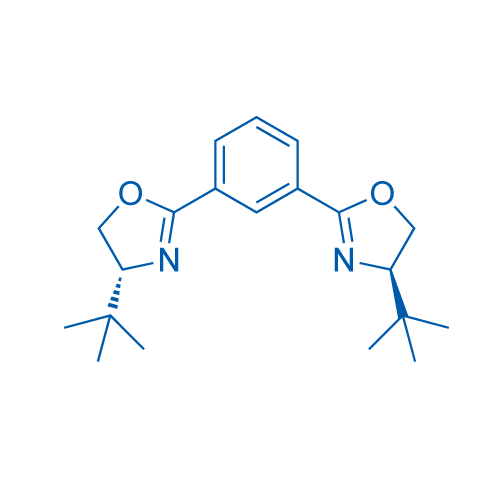
1,3-Bis((R)-4-(tert-butyl)-4,5-dihydrooxazol-2-yl)benzene
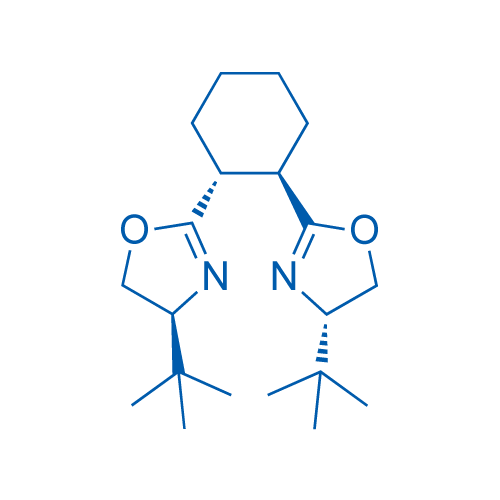
(1R,2R)-1,2-Bis((S)-4-(tert-butyl)-4,5-dihydrooxazol-2-yl)cyclohexane
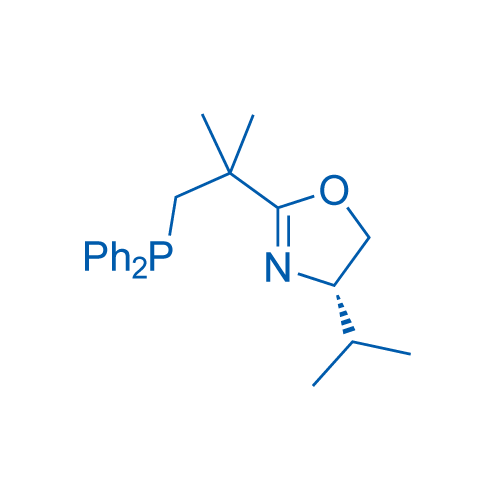
(S)-2-(1-(Diphenylphosphanyl)-2-methylpropan-2-yl)-4-isopropyl-4,5-dihydrooxazole
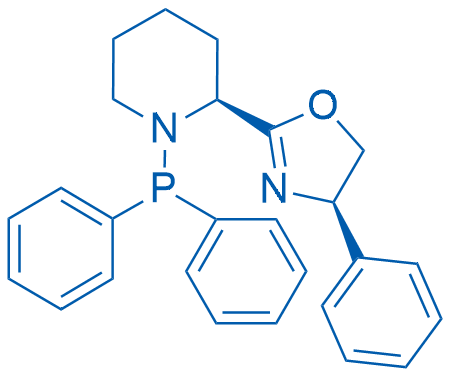
(R)-2-((S)-1-(Diphenylphosphanyl)piperidin-2-yl)-4-phenyl-4,5-dihydrooxazole

(R)-2-(Benzo[b]thiophen-2-yl)-4-isopropyl-4,5-dihydrooxazole

(S)-4-Phenyl-2-(2-((R)-p-tolylsulfinyl)phenyl)-4,5-dihydrooxazole

(R)-4-Cyclohexyl-2-(pyridin-2-yl)-4,5-dihydrooxazole

(R)-4-(tert-Butyl)-2-(1,8-naphthyridin-2-yl)-4,5-dihydrooxazole
Oxazoline ligands currently dominate in asymmetric synthesis, they have been extensively applied in a series of important metal-catalyzed enantioselective reactions.
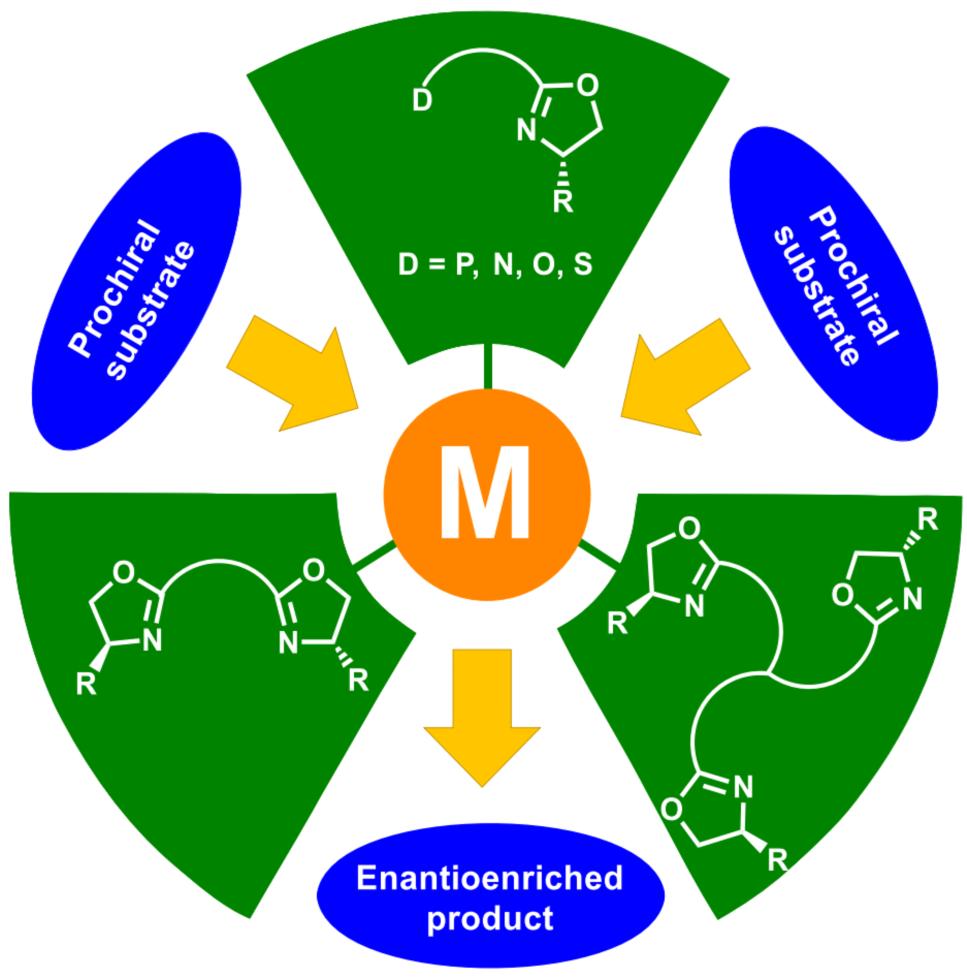
Technical Notes:
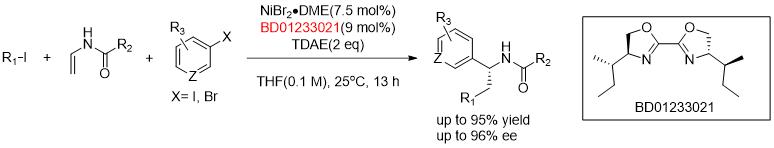
Nickel/Photo-Cocatalyzed Asymmetric Acyl-Carbamoylation of Alkenes.[1]
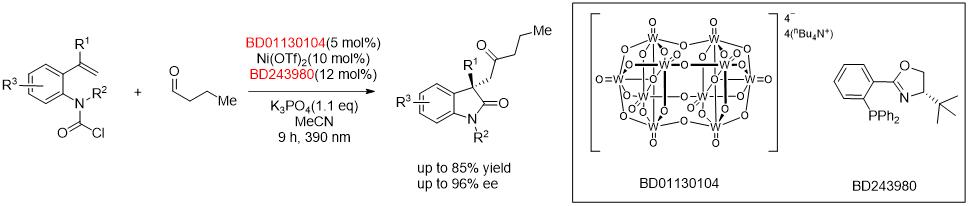
Asymmetric Ni-Catalyzed Radical Relayed Reductive Coupling.[2]
Modular access to substituted cyclohexanes with kinetic stereocontrol.[3]
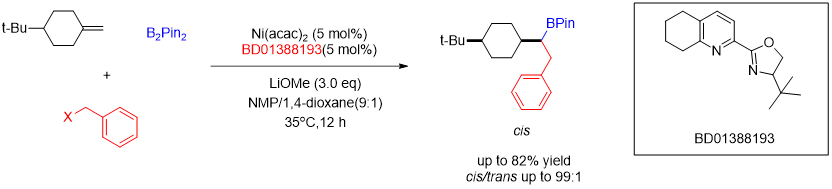
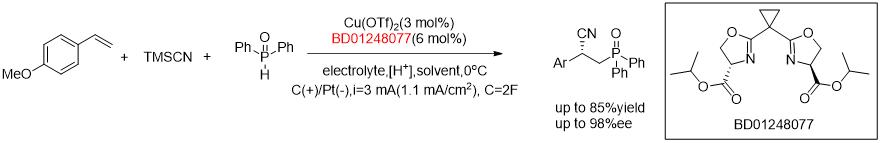
Enantioselective Electrocatalytic Cyanofunctionalization of Vinylarenes.[4]
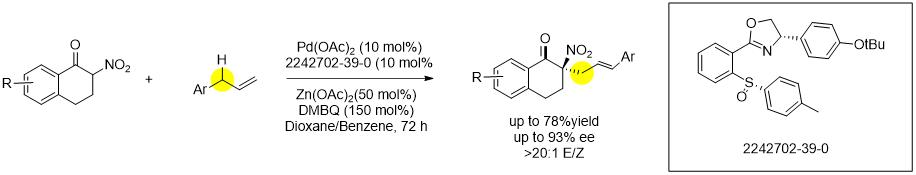
Asymmetric Allylic C-H Alkylation via Palladium(II)/cis-ArSOX Catalysis.[5]
Available Ligands in BLDpharm.
1.PyOX Series
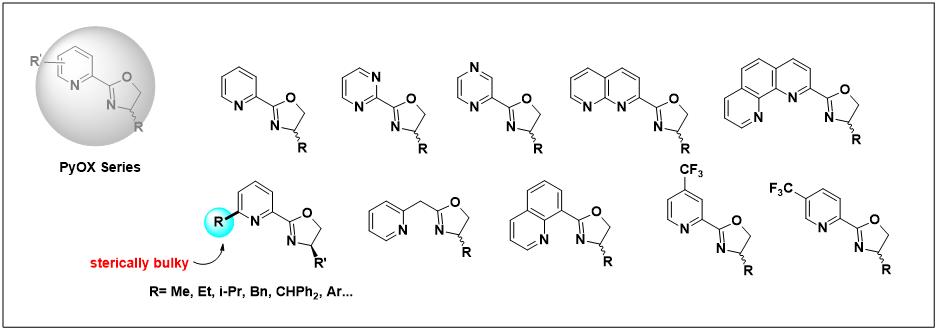
2.BOX Series
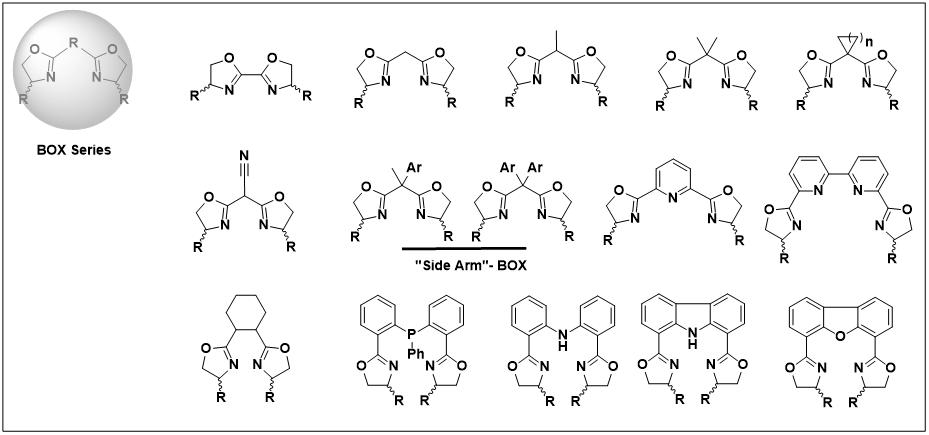
3.PHOX Series
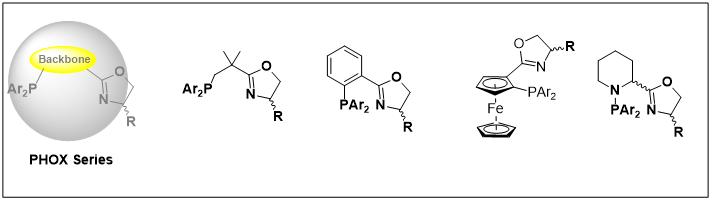
4.SOX Series
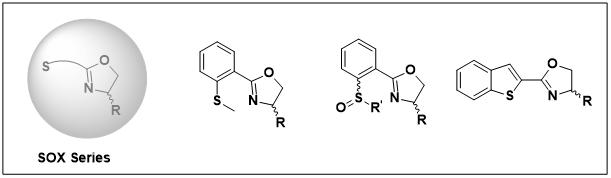
References
[1]J. Am. Chem. Soc. 2020, 142, 2180.
[2]J. Am. Chem. Soc. 2020, 142, 13515.
[3]Science, 2022, 376, 749.
[4]J. Am. Chem. Soc. 2019, 141, 14480.
[5]J. Am. Chem. Soc. 2018, 140, 10658.