BLD Insights
Metal-Organic Frameworks: Carboxylic Acid Ligands
11 July 2022
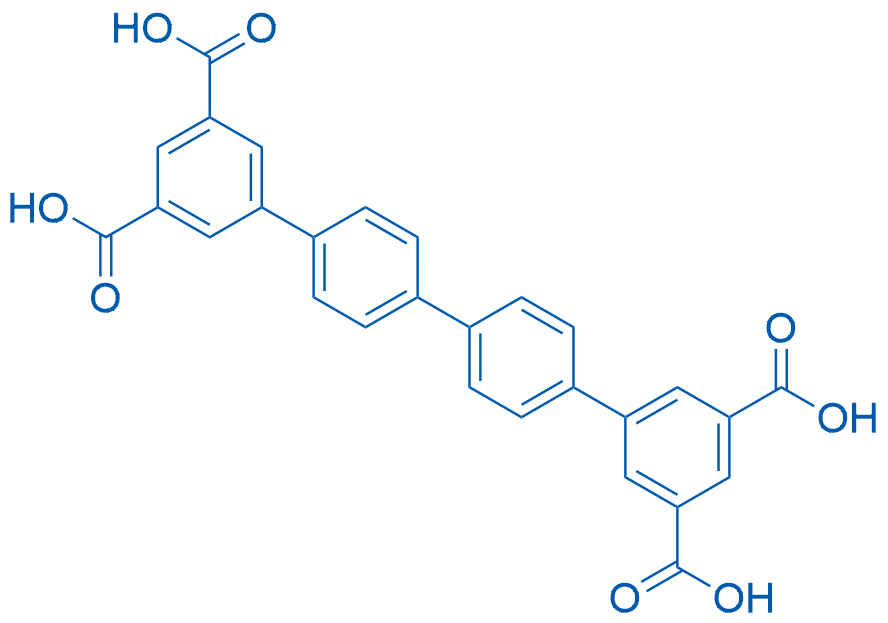
[1,1':4',1'':4'',1'''-Quaterphenyl]-3,3''',5,5'''-tetracarboxylic acid
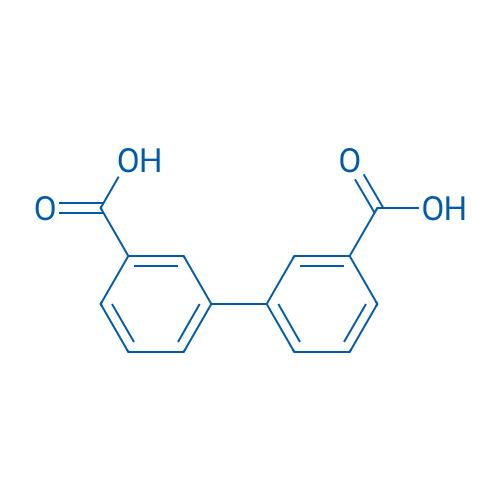
Biphenyl-3,3'-dicarboxylic acid
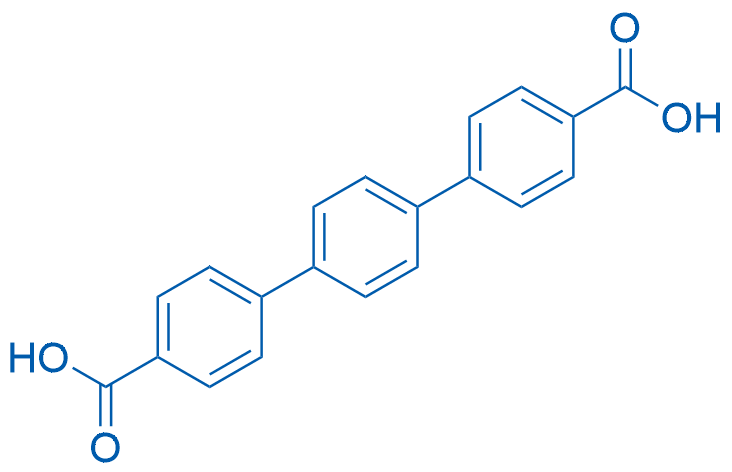
[1,1':4',1''-Terphenyl]-4,4''-dicarboxylic Acid
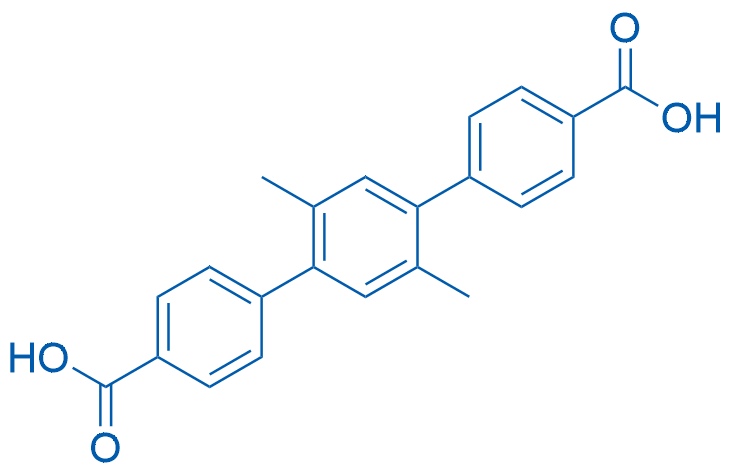
2',5'-Dimethyl-[1,1':4',1''-terphenyl]-4,4''-dicarboxylic acid
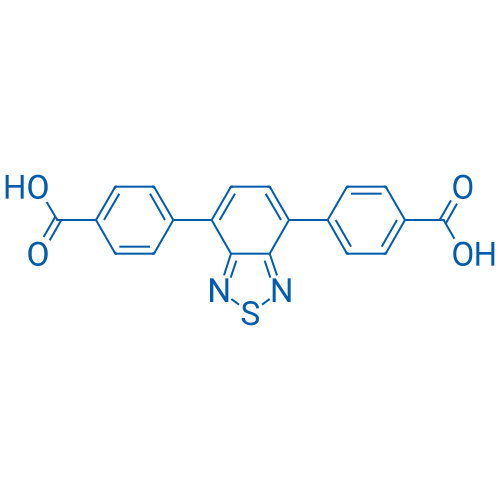
4,4'-(Benzo[c][1,2,5]thiadiazole-4,7-diyl)dibenzoic acid
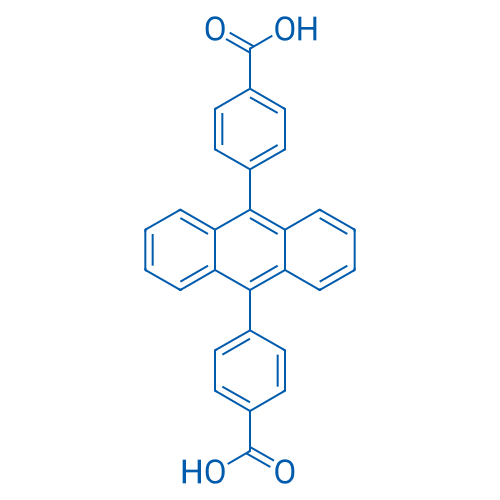
4,4'-(Anthracene-9,10-diyl)dibenzoic acid
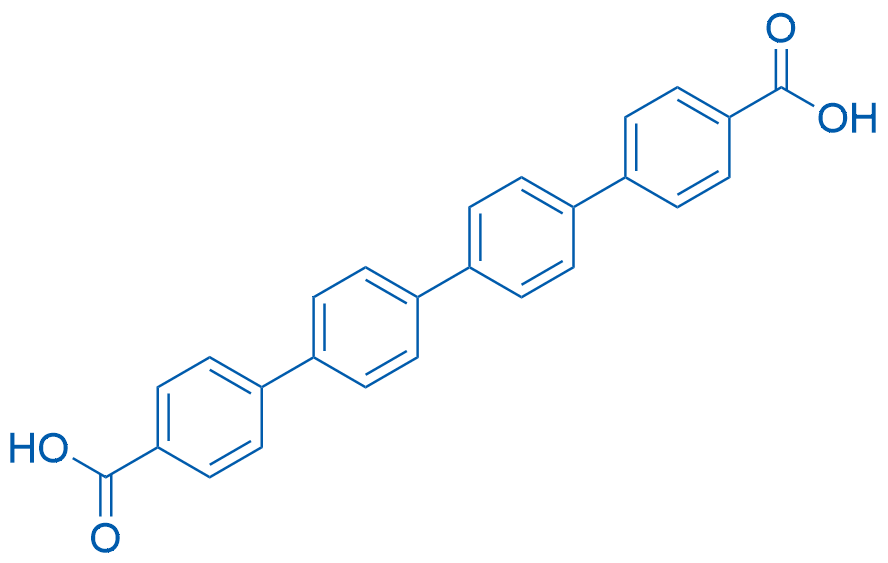
[1,1':4',1'':4'',1'''-Quaterphenyl]-4,4'''-dicarboxylic acid
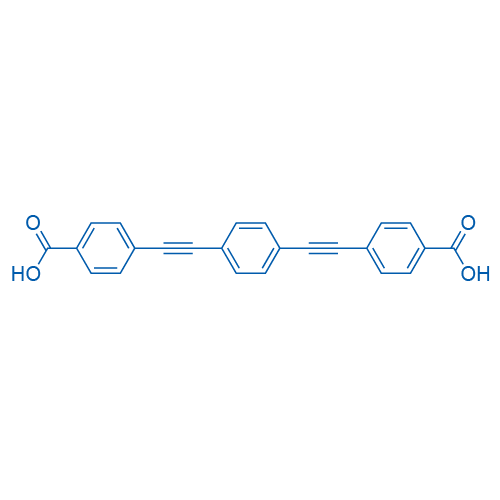
4,4'-[1,4-Phenylenebis(Ethyne-2,1-diyl)]dibenzoic acid
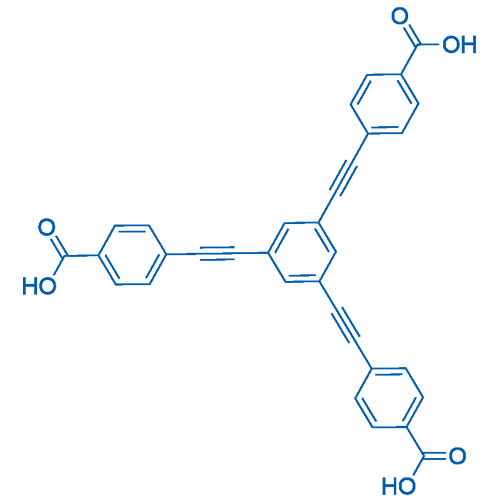
4,4',4''-(Benzene-1,3,5-triyltris(ethyne-2,1-diyl))tribenzoic acid

[1,1':4',1'':4'',1'''-Quaterphenyl]-3,3''',5,5'''-tetracarboxylic acid

Biphenyl-3,3'-dicarboxylic acid

[1,1':4',1''-Terphenyl]-4,4''-dicarboxylic Acid
Metal−organic frameworks (MOFs), an emerging kind of high porous materials, that consist of metal clusters or metal ions and organic ligands, have been extensively studied in the past 3 decades. Because of their unique structural characteristics, including permanent porosity, uniform open cavities, high surface area, adjustable porous size/shape, and modifiable pore size, MOFs have received particular attention in the field of gas storage, separation, catalysis and so on (Figure 1)[1].
Figure 1. MOFs and their applications.
Yaghi and co-workers proposed the concept of MOFs in 1995. Subsequently, in 1999, they synthesized MOF-5 (Figure 2) with large porosity, high specific surface area and good thermal stability from zinc nitrate and terephthalic acid[2]. MOF-5 is a typical MOFs material, and its synthesis and characterization methods are of milestone significance in the field of MOFs research. In the following 20 years, researchers such as Robson, Yaghi and Kitagawa have designed and synthesized a large number of MOFs with novel structures and excellent performance.
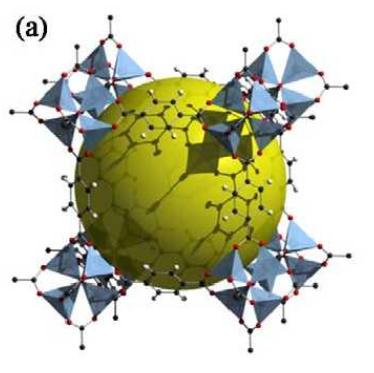
Figure 2. Single-crystal X-ray structures of MOF-5.
On the other hand, organic ligand is an important component of MOFs and a key factor to regulate the structure and properties of MOFs. The structure and properties of MOFs synthesized from organic ligands with similar structures but different substituent groups are also different. Exploring the differences in structure and properties caused by different ligand substituting groups is helpful to realize the directional synthesis of MOFs materials. Carboxylic acid ligands have various coordination modes and are easy to form stable coordination bonds with metal ions. Rigid carboxylic acid ligands tend to form stable pore structures, while flexible carboxylic acid ligands tend to form novel frame structures. The semi-flexible ligand, combining the advantages of the two types of ligand, can realize the structure diversification and functionalization of MOFs materials by changing its own configuration while maintaining the stable channel structure of MOFs.
In 2002, Yaghi and co-workers synthesized a series of MOFs with various pore sizes by the reaction of binary acids and metal ions (Figure 3)[3,4]. Since then, more and more binary, ternary, polycarboxylic acids and their homologous compounds (Figure 4)[5] have been applied to the synthesis of MOFs. Such organic ligands are commonly used in the synthesis of MOFs, and they play an important role in the synthesis of MOFs.
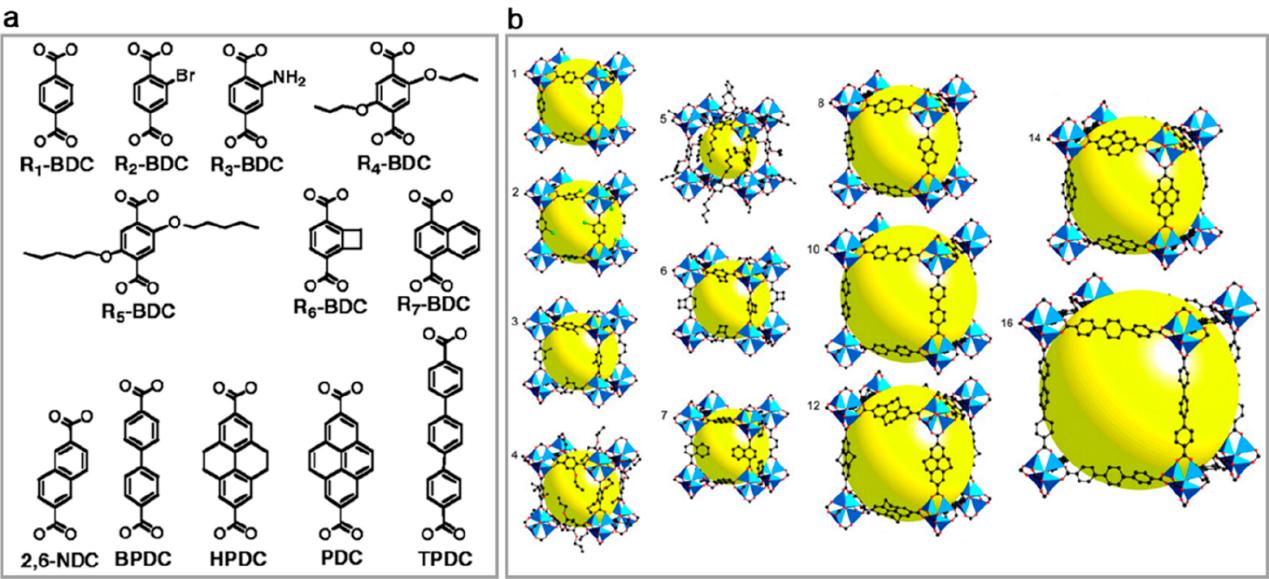
Figure 3. (a) Linear linkers and (b) single-crystal X-ray structures of MOF series.
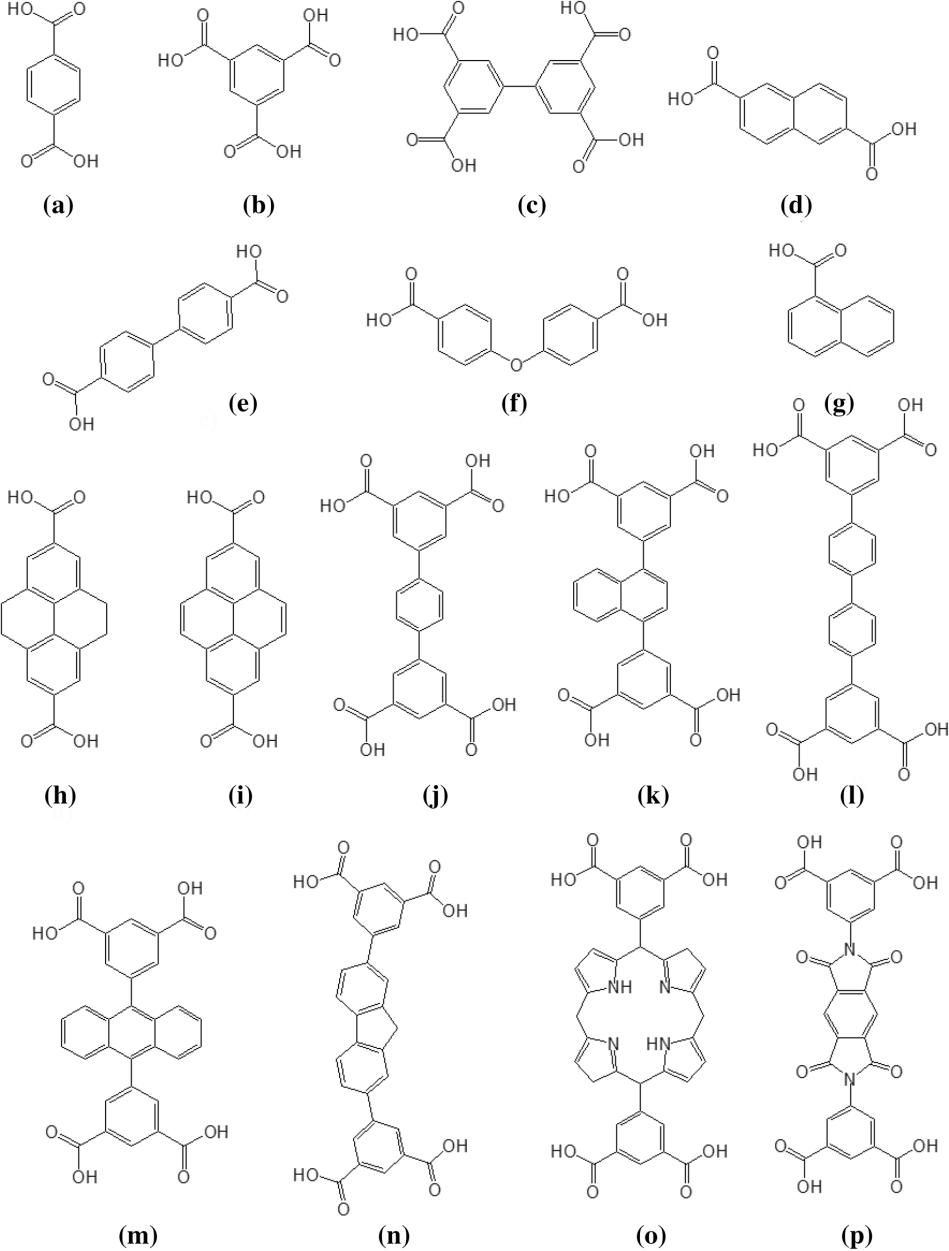
Figure 4. Examples of carboxylic acids.
References
[1]Dzumbira, W.; Ali, N.; Duanmu, C.; Yang, Y.; Khan, A.; Ali, F.; Bilal, M.; Aleya, L.; Iqbal, H. M. N. Separation and remediation of environmental pollutants using metal–organic framework‑based tailored materials. Environ. Sci. Pollut. R. 2022, 29, 4822−4842.
[2]Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O. M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276−279.
[3]Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O. M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469−472.
[4]Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal-rganic framework-based hierarchically porous materials: Synthesis and applications. Chem. Rev. 2021, 121, 12278−12326.
[5]Salehipour, M.; Rezaei, S.; Rezaei, M.; Yazdani, M.; Mogharabi‑Manzari, M. Opportunities and challenges in biomedical applications of metal-organic frameworks. J. Inorg. Organomet. P. Mater. 2021, 31, 4443–4462.