BLD Insights
Suzuki Cross-Coupling - Always Being Your Research Support!
27 July 2022
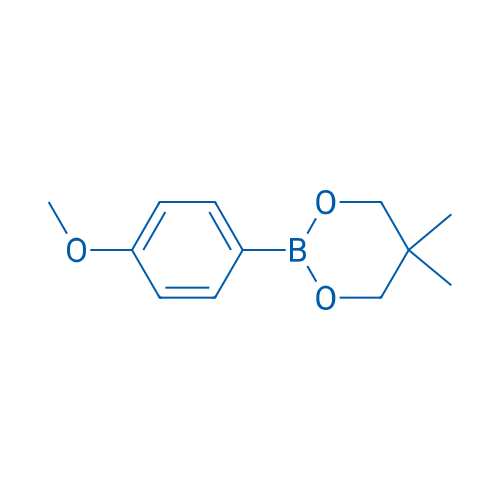
2-(4-Methoxyphenyl)-5,5-dimethyl-1,3,2-dioxaborinane
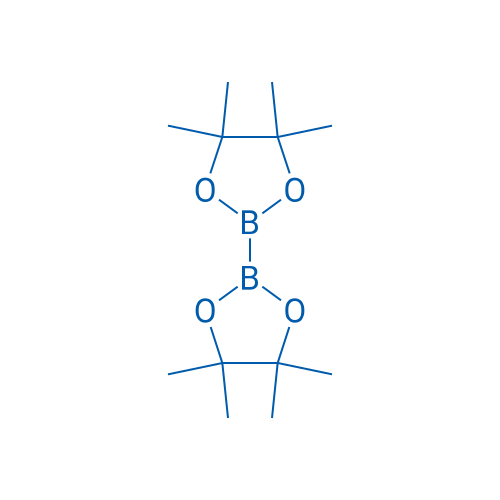
4,4,4',4',5,5,5',5'-Octamethyl-2,2'-bi(1,3,2-dioxaborolane)
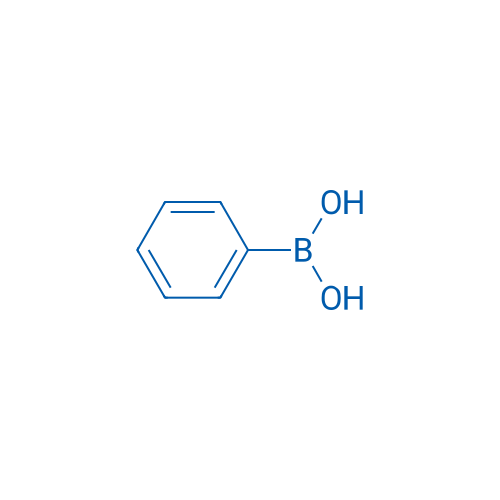
Phenylboronic acid
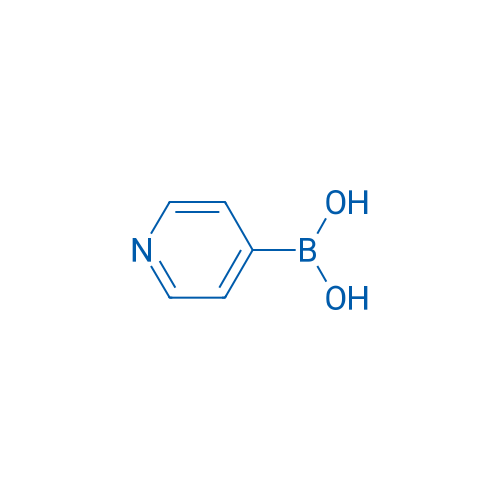
Pyridin-4-ylboronic acid
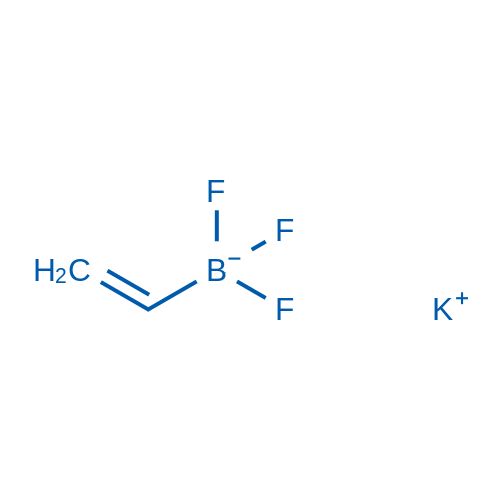
Potassium trifluoro(vinyl)borate
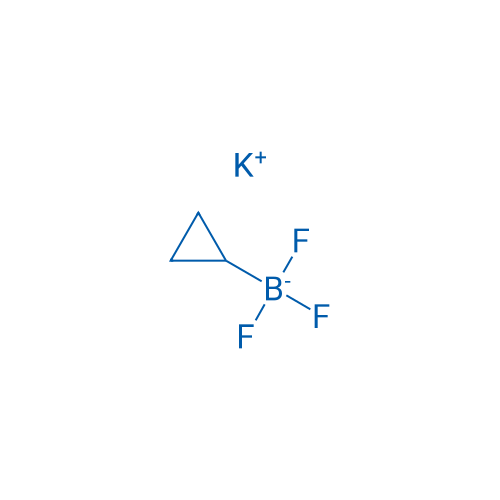
Potassium cyclopropyltrifluoroborate
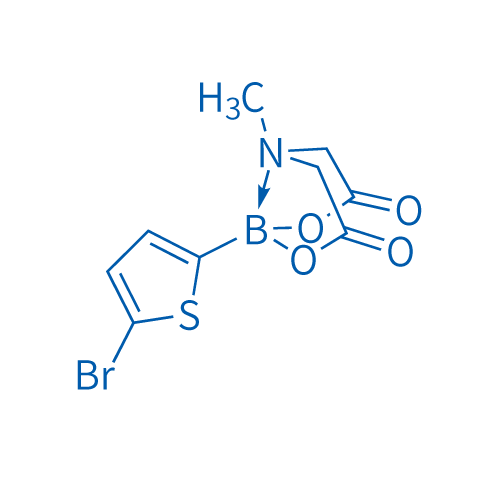
5-Bromo-2-thiophenylboronic acid mida ester
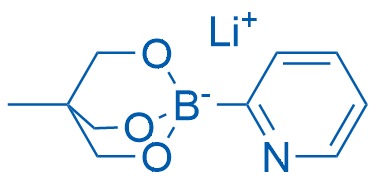
Lithium 4-methyl-1-(pyridin-2-yl)-2,6,7-trioxa-1-borabicyclo[2.2.2]octan-1-uide

2-(4-Methoxyphenyl)-5,5-dimethyl-1,3,2-dioxaborinane

4,4,4',4',5,5,5',5'-Octamethyl-2,2'-bi(1,3,2-dioxaborolane)
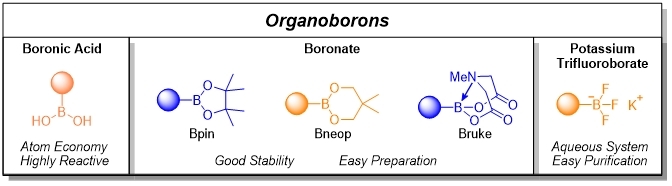
Palladium-catalyzed cross coupling is hailed as one of the most advanced tool available to chemists. Among them, the Suzuki cross-coupling starting with organoboron has a wide range of applications in pharmaceutical chemistry and synthesis of natural products as well as organic materials.
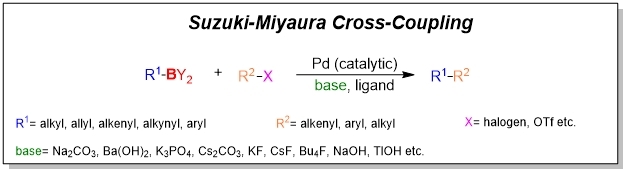
Organoboron nucleophiles consist of boronic acid, potassium trifluoroborate, and boronate. Boronic acids is the most reactive and most atom-economic while boronates such as Bpin and Bneop are stable, cheap and easy preparation. Burke boronates are applied in iterative and automated Suzuki-Miyaura coupling. Potassium trifluoroborate cross-couples efficiently only in the presence of water. All the listed above are suitable for various reaction conditions, showing the difference in reaction activity.