BLD Insights
Potassium Organotrifluoroborates - A Diamond in The Rough
26 August 2022
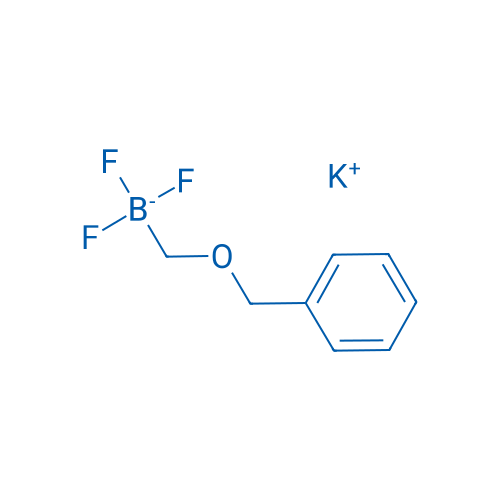
Potassium ((benzyloxy)methyl)trifluoroborate
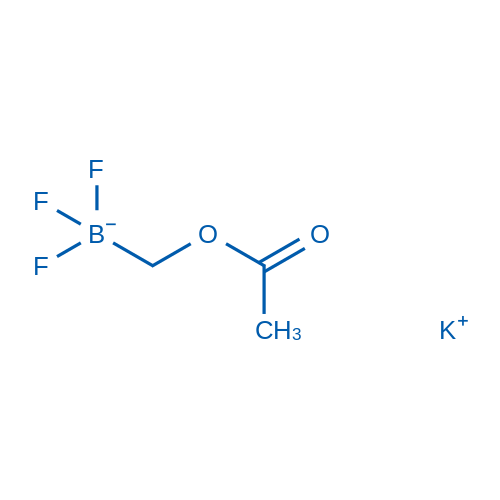
Potassium (acetoxymethyl)trifluoroborate
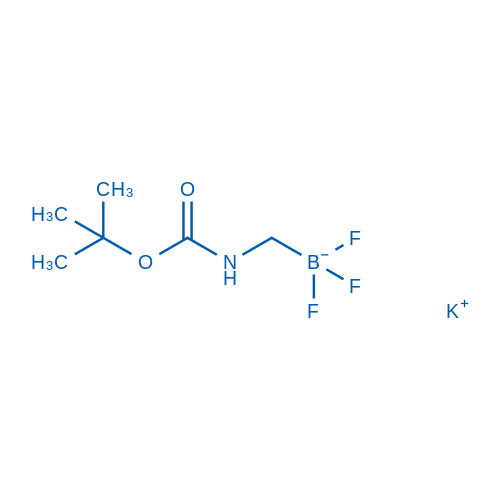
Potassium (((tert-butoxycarbonyl)amino)methyl)trifluoroborate
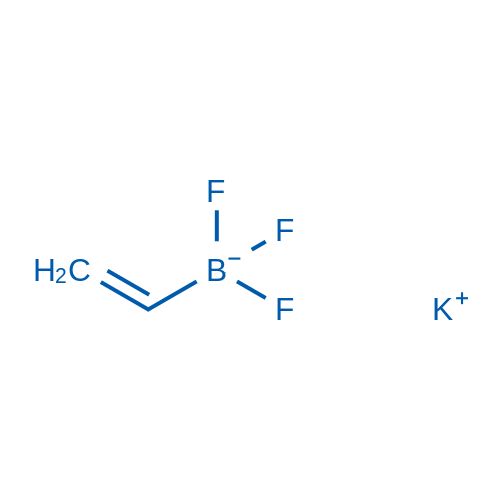
Potassium trifluoro(vinyl)borate
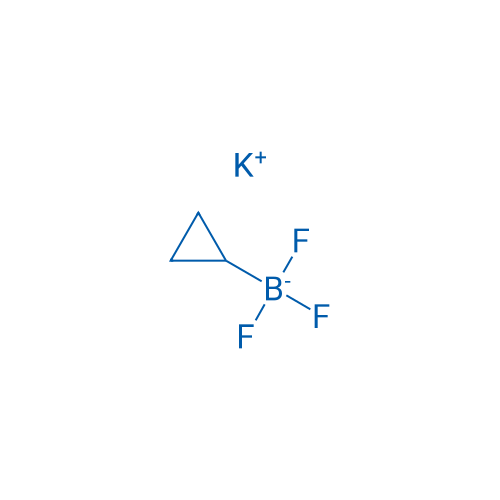
Potassium cyclopropyltrifluoroborate
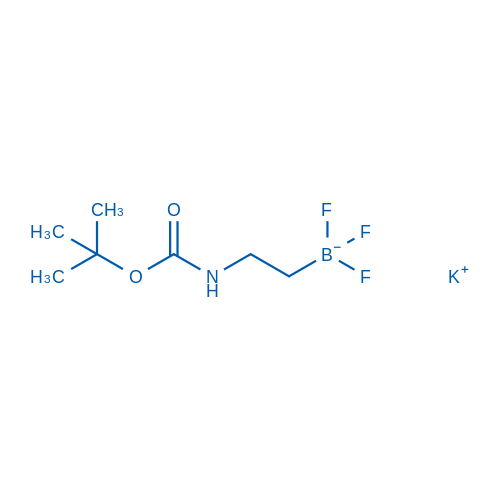
Potassium (2-((tert-butoxycarbonyl)amino)ethyl)trifluoroborate
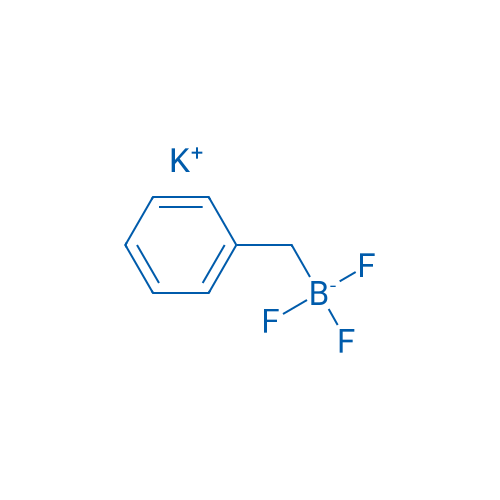
Potassium benzyltrifluoroborate
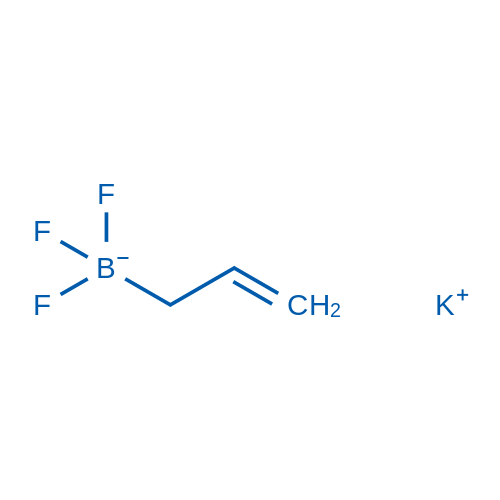
Potassium allyltrifluoroborate
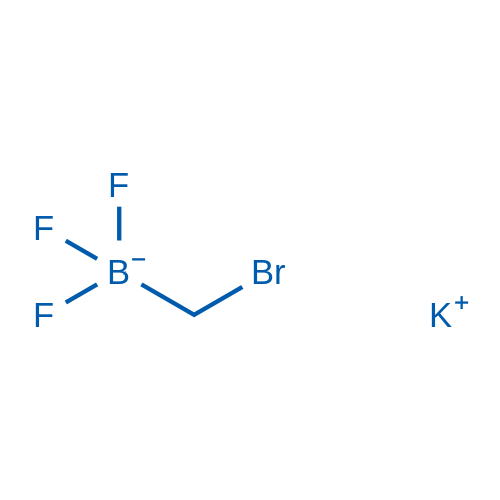
Potassium (bromomethyl)trifluoroborate
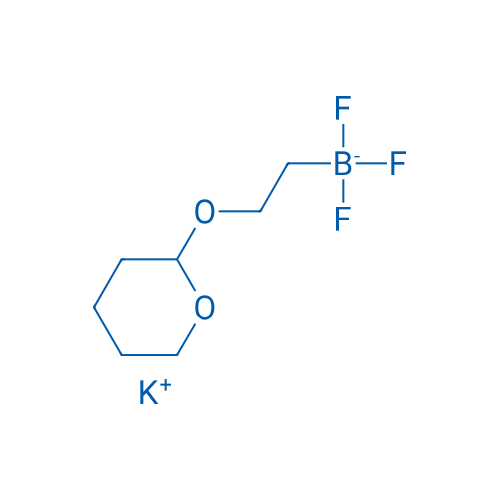
Potassium trifluoro(2-((tetrahydro-2H-pyran-2-yl)oxy)ethyl)borate

Potassium ((benzyloxy)methyl)trifluoroborate

Potassium (acetoxymethyl)trifluoroborate

Potassium (((tert-butoxycarbonyl)amino)methyl)trifluoroborate

Potassium trifluoro(vinyl)borate
Organotrifluoroborates represent an alternative to boronic acids, boronate esters, and organoboranes for use in Suzuki-Miyaura and other transition-metal-catalyzed cross-coupling reactions. The trifluoroborate moiety is stable toward numerous reagents that are often problematic for other boron species. Consequently, remote functional groups within the organotrifluoroborates can be manipulated, while retaining the valuable carbon-boron bond.
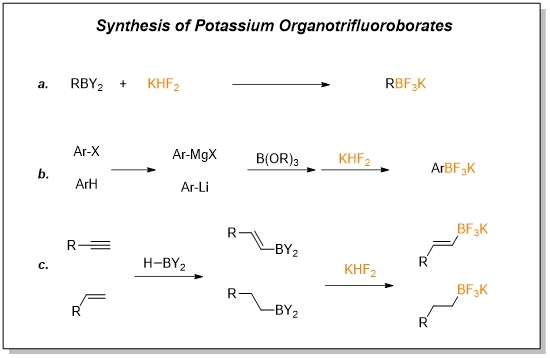
Organotrifluoroborates can be synthesized by a variety of methods, including (a) Trisubstituted organoborons can be converted to related organotrifluoroborates on treatment with KHF2; (b) Lithium-halogen exchange or magnesium insertion followed by boronation and hydrolysis furnished crude boronic acid, which, on treatment with KHF2, gave aryltrifluoroborates; (c) The alkenes or alkynes undergo hydroboration and then react with KHF2 to convert to organotrifluoroborates.
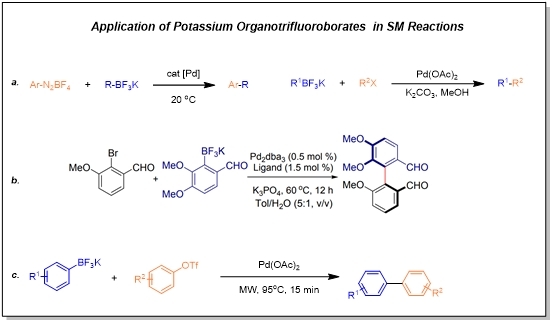
In 1996, the Genet group reported the application of potassium organotrifluoroborates as a nucleophile in SM coupling reactions, gradually attracting the interest of researchers. Subsequent reports include ligand-involved coupling and microwave heating promotion applications. Organotrifluoroborates can overcome some shortcomings of boronic acids and esters and are becoming ideal and promising organoboron nucleophiles.
References
[1]Darses, S.; Genet, J. P. Chem. Rev. 2008, 108, 288-325.
[2]Molander, G. A.; Ellis, N. Acc. Chem. Res. 2007, 40, 275-286
[3]Vedejs, E.; Fields, S. C.; Hayashi, R. J. Am. Chem. Soc. 1999, 121, 2460-2470.
[4]Molander, G. A.; Biolatto, B. Org. Lett. 2002, 4, 1867-1870.
[5]Yang, H.; Sun, J-W.; Gu, W.; Tang, W. J. Am. Chem. Soc. 2020, 142, 8036–8043.
[6]Kabalka, G. W.; Zhou, L.; Naravane, A. Tetrahedon Lett. 2006, 47, 6887-6889.